Biodistribution and safety of a single rAAV3B-AAT vector for silencing and replacement of alpha-1 antitrypsin in Cynomolgus macaques
- PMID: 38445045
- PMCID: PMC10914479
- DOI: 10.1016/j.omtm.2024.101200
Biodistribution and safety of a single rAAV3B-AAT vector for silencing and replacement of alpha-1 antitrypsin in Cynomolgus macaques
Abstract
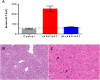
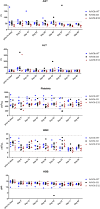
Alpha-1 antitrypsin deficiency (AATD) is characterized by both chronic lung disease due to loss of wild-type AAT (M-AAT) antiprotease function and liver disease due to toxicity from delayed secretion, polymerization, and aggregation of misfolded mutant AAT (Z-AAT). The ideal gene therapy for AATD should therefore comprise both endogenous Z-AAT suppression and M-AAT overexpression. We designed a dual-function rAAV3B (df-rAAV3B) construct, which was effective at transducing hepatocytes, resulting in a considerable decrease of Z-AAT levels and safe M-AAT augmentation in mice. We optimized df-rAAV3B and created two variants, AAV3B-E12 and AAV3B-G3, to simultaneously enhance the concentration of M-AAT in the bloodstream to therapeutic levels and silence endogenous AAT liver expression in cynomolgus monkeys. Our results demonstrate that AAV3b-WT, AAV3B-E12, and AAV3B-G3 were able to transduce the monkey livers and achieve high M-AAT serum levels efficiently and safely. In this nondeficient model, we did not find downregulation of endogenous AAT. However, the dual-function vector did serve as a potentially "liver-sparing" alternative for high-dose liver-mediated AAT gene replacement in the context of underlying liver disease.
Keywords: AAV gene therapy; AAV3B; alpha-1 antitrypsin deficiency; biodistribution; gene silencing; miRNA; preclinical.
© 2024 The Authors.
Conflict of interest statement
T.R.F., A.G., C.M., and A.K. report financial support was provided by National Heart Lung and Blood Institute. T.R.F. and A.G. report equipment, drugs, or supplies was provided by National Heart Lung and Blood Institute Gene Therapy Resource Program. C.M. reports a relationship with Sanofi that includes: employment. C.M. reports a relationship with Apic Bio that includes: board membership and equity or stocks. T.R.F. and C.M. have a patent pending to Apic Bio. T.R.F. is Chair of the NHLBI Gene Therapy Resource Program Advisory Committee. C.M. is a named inventor on the patent for the dual function AAT construct; D.M., S.Z., and C.M. are named inventors on the patent for the AAV3B variants.
Figures







Similar articles
-
Hepatocyte proteomes reveal the role of protein disulfide isomerase 4 in alpha 1-antitrypsin deficiency.JHEP Rep. 2021 Apr 24;3(4):100297. doi: 10.1016/j.jhepr.2021.100297. eCollection 2021 Aug. JHEP Rep. 2021. PMID: 34151245 Free PMC article.
-
Survival Advantage of Both Human Hepatocyte Xenografts and Genome-Edited Hepatocytes for Treatment of α-1 Antitrypsin Deficiency.Mol Ther. 2017 Nov 1;25(11):2477-2489. doi: 10.1016/j.ymthe.2017.09.020. Epub 2017 Sep 25. Mol Ther. 2017. PMID: 29032169 Free PMC article.
-
Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles.Mol Ther. 2012 Mar;20(3):590-600. doi: 10.1038/mt.2011.292. Epub 2012 Jan 17. Mol Ther. 2012. PMID: 22252449 Free PMC article.
-
Alpha1-antitrypsin deficiency: New therapies on the horizon.Curr Opin Pharmacol. 2021 Aug;59:149-156. doi: 10.1016/j.coph.2021.06.001. Epub 2021 Jul 10. Curr Opin Pharmacol. 2021. PMID: 34256305 Review.
-
Challenges and Prospects for Alpha-1 Antitrypsin Deficiency Gene Therapy.Hum Gene Ther. 2015 Nov;26(11):709-18. doi: 10.1089/hum.2015.044. Epub 2015 Sep 29. Hum Gene Ther. 2015. PMID: 26413996 Free PMC article. Review.
Cited by
-
Limb Perfusion Delivery of a rAAV1 Alpha-1 Antitrypsin Vector in Non-Human Primates Is Safe but Insufficient for Therapy.Genes (Basel). 2024 Sep 10;15(9):1188. doi: 10.3390/genes15091188. Genes (Basel). 2024. PMID: 39336779 Free PMC article.
-
A single amino acid variant in the variable region I of AAV capsid confers liver detargeting.bioRxiv [Preprint]. 2025 Jun 14:2025.03.04.641478. doi: 10.1101/2025.03.04.641478. bioRxiv. 2025. PMID: 40093067 Free PMC article. Preprint.
References
-
- Brantly M.L., Chulay J.D., Wang L., Mueller C., Humphries M., Spencer L.T., Rouhani F., Conlon T.J., Calcedo R., Betts M.R., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. - DOI - PMC - PubMed
-
- Flotte T.R., Trapnell B.C., Humphries M., Carey B., Calcedo R., Rouhani F., Campbell-Thompson M., Yachnis A.T., Sandhaus R.A., McElvaney N.G., et al. Phase 2 Clinical Trial of a Recombinant Adeno-Associated Viral Vector Expressing α 1 -Antitrypsin: Interim Results. Hum. Gene Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. - DOI - PMC - PubMed
-
- Mueller C., Chulay J.D., Trapnell B.C., Humphries M., Carey B., Sandhaus R.A., McElvaney N.G., Messina L., Tang Q., Rouhani F.N., et al. Human Treg responses allow sustained recombinant adeno-associated virus–mediated transgene expression. J. Clin. Invest. 2013;123:5310–5318. doi: 10.1172/JCI70314. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

