Penehyclidine for Prevention of Postoperative Nausea and Vomiting in Patients Undergoing Gynecological Laparoscopic Surgery Under Combined Intravenous and Inhalation Anesthesia: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 38445065
- PMCID: PMC10913797
- DOI: 10.2147/DDDT.S453327
Penehyclidine for Prevention of Postoperative Nausea and Vomiting in Patients Undergoing Gynecological Laparoscopic Surgery Under Combined Intravenous and Inhalation Anesthesia: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Purpose: We designed this study to investigate the effect of intravenous use of penehyclidine on postoperative nausea and vomiting (PONV) after gynecological laparoscopic surgery.
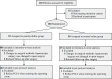
Patients and methods: Ninety-two Women Patients (Aged ≥ 18) Scheduled for Elective Gynecologic Laparoscopy Were Enrolled in the Current Study. Patients Were Equally Randomized Assigned Into Penehyclidine group (PHC group: received a bolus of penehyclidine 10 μg/kg during the induction of anesthesia, then followed by a continuous infusion of 10 μg/kg penehyclidine at a fixed rate of 2.0 mL/h in postoperative intravenous analgesia pump over 48h, 0.5 mg upper limit respectively) or Control group (received 0.9% saline in replace of penehyclidine at the same time points). The primary outcome measure was the incidence of postoperative nausea and vomiting in the postanesthesia care unit and ward area. Quality of Recovery-15 (QoR-15) scores and general comfort questionnaire (GCQ) scores were assessed on postoperative day (POD) 1, 2.
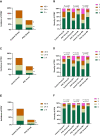
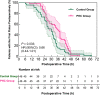
Results: Patients between two groups had comparable baseline characteristics. Compared with the Control group, the incidence and severity of PONV, postoperative nausea (PON), and postoperative vomiting (POV) were significantly lower in the PHC group at 2h (PONV: P = 0.002, P = 0.004, respectively; PON: P = 0.018, P = 0.038, respectively; POV: P = 0.011, P = 0.072, respectively), 24h (PONV: P = 0.003, P = 0.001, respectively; PON: P = 0.010, P = 0.032, respectively; POV: P = 0.006, P = 0.044, respectively), and 48h (PONV: P = 0.003, P = 0.002, respectively; PON: P = 0.007, P = 0.019, respectively; POV: P = 0.002, P = 0.013, respectively) after surgery. The QoR-15 and GCQ scores of the PHC group were significantly higher than those of the Control group at POD 1, 2 (P < 0.001; P < 0.001, respectively).
Conclusion: Our findings suggest that perioperative intravenous application of penehyclidine can effectively prevent postoperative nausea and vomiting in gynecological laparoscopic surgery patients and improve postoperative recovery.
Keywords: gynecologic laparoscopy; penehyclidine; postoperative nausea and vomiting; quality of recovery.
© 2024 Zhao et al.
Conflict of interest statement
All authors have no conflicts of interest in this work.
Figures
Similar articles
-
Study of penehyclidine for the prevention of postoperative nausea and vomiting following laparoscopic sleeve gastrectomy under general anesthesia: a randomized, prospective, double-blind trial.J Anesth. 2025 Feb;39(1):67-74. doi: 10.1007/s00540-024-03424-3. Epub 2024 Nov 11. J Anesth. 2025. PMID: 39528821 Clinical Trial.
-
Penehyclidine enhances the efficacy of tropisetron in prevention of PONV following gynecological laparoscopic surgery.J Anesth. 2012 Dec;26(6):864-9. doi: 10.1007/s00540-012-1443-1. Epub 2012 Aug 10. J Anesth. 2012. PMID: 22878869 Clinical Trial.
-
Penehyclidine hydrochloride for treating postoperative nausea and vomiting after laparoscopic bariatric surgery: a double-blinded randomized controlled trial.BMC Anesthesiol. 2023 Apr 24;23(1):135. doi: 10.1186/s12871-023-02078-0. BMC Anesthesiol. 2023. PMID: 37095439 Free PMC article. Clinical Trial.
-
Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting.Cochrane Database Syst Rev. 2019 Mar 29;3(3):CD012212. doi: 10.1002/14651858.CD012212.pub2. Cochrane Database Syst Rev. 2019. PMID: 30925195 Free PMC article.
-
Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: A meta-analysis of randomized controlled trials.Int J Surg. 2020 Jan;73:57-64. doi: 10.1016/j.ijsu.2019.10.036. Epub 2019 Nov 6. Int J Surg. 2020. PMID: 31704425 Review.
Cited by
-
Penehyclidine for Prevention of Postoperative Nausea and Vomiting in Patients Undergoing Gynecological Laparoscopic Surgery Under Combined Intravenous and Inhalation Anesthesia: A Randomized, Double-Blind, Placebo-Controlled Trial [Letter].Drug Des Devel Ther. 2024 Jun 17;18:2299-2300. doi: 10.2147/DDDT.S482220. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38911028 Free PMC article. Clinical Trial. No abstract available.
-
Penehyclidine combined with antiemetics for preventing postoperative nausea and vomiting: A meta-analysis of randomized control trials and trial sequential analysis.Medicine (Baltimore). 2025 Jun 20;104(25):e42908. doi: 10.1097/MD.0000000000042908. Medicine (Baltimore). 2025. PMID: 40550034 Free PMC article.
-
Study of penehyclidine for the prevention of postoperative nausea and vomiting following laparoscopic sleeve gastrectomy under general anesthesia: a randomized, prospective, double-blind trial.J Anesth. 2025 Feb;39(1):67-74. doi: 10.1007/s00540-024-03424-3. Epub 2024 Nov 11. J Anesth. 2025. PMID: 39528821 Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous