Assessing Longitudinal Treatment Efficacies and Alterations in Molecular Markers Associated with Glutamatergic Signaling and Immune Checkpoint Inhibitors in a Spontaneous Melanoma Mouse Model
- PMID: 38445232
- PMCID: PMC10914525
- DOI: 10.1016/j.xjidi.2024.100262
Assessing Longitudinal Treatment Efficacies and Alterations in Molecular Markers Associated with Glutamatergic Signaling and Immune Checkpoint Inhibitors in a Spontaneous Melanoma Mouse Model
Abstract
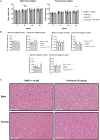
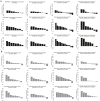
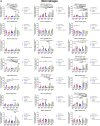
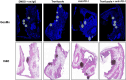
Previous work done by our laboratory described the use of an immunocompetent spontaneous melanoma-prone mouse model, TGS (TG-3/SKH-1), to evaluate treatment outcomes using inhibitors of glutamatergic signaling and immune checkpoint for 18 weeks. We showed a significant therapeutic efficacy with a notable sex-biased response in male mice. In this follow-up 18-week study, the dose of the glutamatergic signaling inhibitor was increased (from 1.7 mg/kg to 25 mg/kg), which resulted in improved responses in female mice but not male mice. The greatest reduction in tumor progression was observed in male mice treated with single-agent troriluzole and anti-PD-1. Furthermore, a randomly selected group of mice was removed from treatment after 18 weeks and maintained for up to an additional 48 weeks demonstrating the utility of the TGS mouse model to perform a ≥1-year preclinical therapeutic study in a physiologically relevant tumor-host environment. Digital spatial imaging analyses were performed in tumors and tumor microenvironments across treatment modalities using antibody panels for immune cell types and immune cell activation. The results suggest that immune cell populations and cytotoxic activities of T cells play critical roles in treatment responses in these mice. Examination of a group of molecular protein markers based on the proposed mechanisms of action of inhibitors of glutamatergic signaling and immune checkpoint showed that alterations in expression levels of xCT, γ-H2AX, EAAT2, PD-L1, and PD-1 are likely associated with the loss of treatment responses. These results suggest the importance of tracking changes in molecular markers associated with the mechanism of action of therapeutics over the course of a longitudinal preclinical therapeutic study in spatial and temporal manners.
Keywords: Cancer biology; Drug development; Melanoma; Methods/tools/techniques; Mouse models.
© 2024 The Authors.
Figures












References
-
- Barr J.T., Tran T.B., Rock B.M., Wahlstrom J.L., Dahal U.P. Strain-dependent variability of early discovery small molecule pharmacokinetics in mice: does strain matter? Drug Metab Dispos. 2020;48:613–621. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

