Beneficial Effects of Human Schwann Cell-Derived Exosomes in Mitigating Secondary Damage After Penetrating Ballistic-Like Brain Injury
- PMID: 38445369
- PMCID: PMC11631803
- DOI: 10.1089/neu.2023.0650
Beneficial Effects of Human Schwann Cell-Derived Exosomes in Mitigating Secondary Damage After Penetrating Ballistic-Like Brain Injury
Abstract
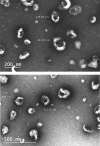
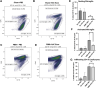
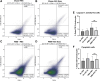
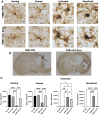
There is a growing body of evidence that the delivery of cell-derived exosomes normally involved in intracellular communication can reduce secondary injury mechanisms after brain and spinal cord injury and improve outcomes. Exosomes are nanometer-sized vesicles that are released by Schwann cells and may have neuroprotective effects by reducing post-traumatic inflammatory processes as well as promoting tissue healing and functional recovery. The purpose of this study was to evaluate the beneficial effects of human Schwann-cell exosomes (hSC-Exos) in a severe model of penetrating ballistic-like brain injury (PBBI) in rats and investigate effects on multiple outcomes. Human Schwann cell processing protocols followed Current Good Manufacturing Practices (cGMP) with exosome extraction and purification steps approved by the Food and Drug Administration for an expanded access single ALS patient Investigational New Drug. Anesthetized male Sprague-Dawley rats (280-350g) underwent PBBI surgery or Sham procedures and, starting 30 min after injury, received either a dose of hSC-Exos or phosphate-buffered saline through the jugular vein. At 48h after PBBI, flow cytometry analysis of cortical tissue revealed that hSC-Exos administration reduced the number of activated microglia and levels of caspase-1, a marker of inflammasome activation. Neuropathological analysis at 21 days showed that hSC-Exos treatment after PBBI significantly reduced overall contusion volume and decreased the frequency of Iba-1 positive activated and amoeboid microglia by immunocytochemical analysis. This study revealed that the systemic administration of hSC-Exos is neuroprotective in a model of severe TBI and reduces secondary inflammatory injury mechanisms and histopathological damage. The administration of hSC-Exos represents a clinically relevant cell-based therapy to limit the detrimental effects of neurotrauma or other progressive neurological injuries by impacting multiple pathophysiological events and promoting neurological recovery.
Keywords: exosomes; human Schwann cells; inflammasome; inflammation; penetrating ballistic-like brain injury; traumatic brain injury.
Figures




Similar articles
-
Human Schwann cell exosome treatment attenuates secondary injury mechanisms, histopathological consequences, and behavioral deficits after traumatic brain injury.Neurotherapeutics. 2025 Apr;22(3):e00555. doi: 10.1016/j.neurot.2025.e00555. Epub 2025 Feb 22. Neurotherapeutics. 2025. PMID: 39988499 Free PMC article.
-
The role of microglial inflammasome activation in pyroptotic cell death following penetrating traumatic brain injury.J Neuroinflammation. 2019 Feb 8;16(1):27. doi: 10.1186/s12974-019-1423-6. J Neuroinflammation. 2019. PMID: 30736791 Free PMC article.
-
Microglial Inflammasome Activation in Penetrating Ballistic-Like Brain Injury.J Neurotrauma. 2018 Jul 15;35(14):1681-1693. doi: 10.1089/neu.2017.5530. Epub 2018 Apr 2. J Neurotrauma. 2018. PMID: 29439605 Free PMC article.
-
Therapeutic effect of exosomes derived from Schwann cells in the repair of peripheral nerve injury.Life Sci. 2024 Nov 15;357:123086. doi: 10.1016/j.lfs.2024.123086. Epub 2024 Sep 30. Life Sci. 2024. PMID: 39357794 Review.
-
Regulation of nerve cells and therapeutic potential in central nervous system injury using microglia-derived exosomes.Neuroscience. 2024 Dec 17;563:84-92. doi: 10.1016/j.neuroscience.2024.11.011. Epub 2024 Nov 8. Neuroscience. 2024. PMID: 39521323 Review.
Cited by
-
Extracellular vesicles derived from Schwann cells to enhance bone and dental tissue regeneration: a literature review.J Nanobiotechnology. 2025 Jul 11;23(1):502. doi: 10.1186/s12951-025-03585-7. J Nanobiotechnology. 2025. PMID: 40646600 Free PMC article. Review.
-
Extracellular vesicles as drug and gene delivery vehicles in central nervous system diseases.Biomater Sci. 2025 Feb 25;13(5):1161-1178. doi: 10.1039/d4bm01394h. Biomater Sci. 2025. PMID: 39871579 Free PMC article. Review.
-
Human Schwann Cell-Derived Extracellular Vesicle Isolation, Bioactivity Assessment, and Omics Characterization.Int J Nanomedicine. 2025 Apr 4;20:4123-4144. doi: 10.2147/IJN.S500159. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40201152 Free PMC article.
-
Open-Field Blast Injury Disrupts Corneal Gene Expression Linked to Ion Transport, Sensory Perception, and Neural Signaling.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):68. doi: 10.1167/iovs.66.11.68. Invest Ophthalmol Vis Sci. 2025. PMID: 40862667 Free PMC article.
-
Neuroprotective Mechanism of MOTS-c in TBI Mice: Insights from Integrated Transcriptomic and Metabolomic Analyses.Drug Des Devel Ther. 2024 Jul 15;18:2971-2987. doi: 10.2147/DDDT.S460265. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39050800 Free PMC article.
References
-
- Hyder AA, Wunderlich CA, Puvanachandra P, et al. . The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007;22(5):341–353 - PubMed
-
- Johnson WD, Griswold DP. Traumatic brain injury: a global challenge. Lancet Neurol 2017;16(12):949–950 - PubMed
-
- Tasigiorgos S, Economopoulos KP, Winfield RD, et al. . Firearm injury in the United States: an overview of an evolving public health problem. J Am Coll Surg 2015;221(6):1005–1014 - PubMed
-
- Khan MB, Kumar R, Irfan FB, et al. . Civilian craniocerebral gunshot injuries in a developing country: presentation, injury characteristics, prognostic indicators, and complications. World Neurosurg 2014;82(1-2):14–19 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
