Ovariectomy-Induced Arterial Stiffening Differs From Vascular Aging and Is Reversed by GPER Activation
- PMID: 38445498
- PMCID: PMC11023783
- DOI: 10.1161/HYPERTENSIONAHA.123.22024
Ovariectomy-Induced Arterial Stiffening Differs From Vascular Aging and Is Reversed by GPER Activation
Abstract
Background: Arterial stiffness is a cardiovascular risk factor and dramatically increases as women transition through menopause. The current study assessed whether a mouse model of menopause increases arterial stiffness in a similar manner to aging and whether activation of the G-protein-coupled estrogen receptor could reverse stiffness.
Methods: Female C57Bl/6J mice were ovariectomized at 10 weeks of age or aged to 52 weeks, and some mice were treated with G-protein-coupled estrogen receptor agonists.
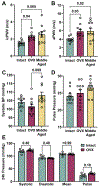
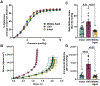
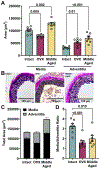
Results: Ovariectomy and aging increased pulse wave velocity to a similar extent independent of changes in blood pressure. Aging increased carotid wall thickness, while ovariectomy increased material stiffness without altering vascular geometry. RNA-sequencing analysis revealed that ovariectomy downregulated smooth muscle contractile genes. The enantiomerically pure G-protein-coupled estrogen receptor agonist, LNS8801, reversed stiffness in ovariectomy mice to a greater degree than the racemic agonist G-1. In summary, ovariectomy and aging induced arterial stiffening via potentially different mechanisms. Aging was associated with inward remodeling, while ovariectomy-induced material stiffness independent of geometry and a loss of the contractile phenotype.
Conclusions: This study enhances our understanding of the impact of estrogen loss on vascular health in a murine model and warrants further studies to examine the ability of LNS8801 to improve vascular health in menopausal women.
Keywords: aging; estrogens; menopause; mice; vascular stiffness.
Conflict of interest statement
Figures



Update of
-
Ovariectomy-Induced Arterial Stiffening Differs from Vascular Aging and is Reversed by GPER Activation.bioRxiv [Preprint]. 2023 Aug 14:2023.08.10.552881. doi: 10.1101/2023.08.10.552881. bioRxiv. 2023. Update in: Hypertension. 2024 May;81(5):e51-e62. doi: 10.1161/HYPERTENSIONAHA.123.22024. PMID: 37645992 Free PMC article. Updated. Preprint.
References
-
- Oh YS, Berkowitz DE, Cohen RA, Figueroa CA, Harrison DG, Humphrey JD, Larson DF, Leopold JA, Mecham RP, Ruiz-Opazo N, et al. A special report on the NHLBI initiative to study cellular and molecular mechanisms of arterial stiffness and its association with hypertension. Circulation Research. 2017;121:1216–1218. doi: 10.1161/CIRCRESAHA.117.311703 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

