Effects of dopamine and opioid receptor antagonism on the neural processing of social and nonsocial rewards
- PMID: 38445523
- PMCID: PMC10915723
- DOI: 10.1002/hbm.26645
Effects of dopamine and opioid receptor antagonism on the neural processing of social and nonsocial rewards
Abstract
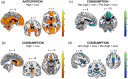
Rewards are a broad category of stimuli inducing approach behavior to aid survival. Extensive evidence from animal research has shown that wanting (the motivation to pursue a reward) and liking (the pleasure associated with its consumption) are mostly regulated by dopaminergic and opioidergic activity in dedicated brain areas. However, less is known about the neuroanatomy of dopaminergic and opioidergic regulation of reward processing in humans, especially when considering different types of rewards (i.e., social and nonsocial). To fill this gap of knowledge, we combined dopaminergic and opioidergic antagonism (via amisulpride and naltrexone administration) with functional neuroimaging to investigate the neurochemical and neuroanatomical bases of wanting and liking of matched nonsocial (food) and social (interpersonal touch) rewards, using a randomized, between-subject, placebo-controlled, double-blind design. While no drug effect was observed at the behavioral level, brain activity was modulated by the administered compounds. In particular, opioid antagonism, compared to placebo, reduced activity in the medial orbitofrontal cortex during consumption of the most valued social and nonsocial rewards. Dopamine antagonism, however, had no clear effects on brain activity in response to reward anticipation. These findings provide insights into the neurobiology of human reward processing and suggest a similar opioidergic regulation of the neural responses to social and nonsocial reward consumption.
Keywords: dopamine; fMRI; food; opioids; reward; social touch.
© 2024 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards.Elife. 2020 Oct 13;9:e55797. doi: 10.7554/eLife.55797. Elife. 2020. PMID: 33046213 Free PMC article.
-
Facial responses of adult humans during the anticipation and consumption of touch and food rewards.Cognition. 2020 Jan;194:104044. doi: 10.1016/j.cognition.2019.104044. Epub 2019 Sep 6. Cognition. 2020. PMID: 31499297
-
Opioid antagonism modulates wanting-related frontostriatal connectivity.Elife. 2021 Nov 11;10:e71077. doi: 10.7554/eLife.71077. Elife. 2021. PMID: 34761749 Free PMC article. Clinical Trial.
-
Evaluation of the Social Motivation Hypothesis of Autism: A Systematic Review and Meta-analysis.JAMA Psychiatry. 2018 Aug 1;75(8):797-808. doi: 10.1001/jamapsychiatry.2018.1100. JAMA Psychiatry. 2018. PMID: 29898209 Free PMC article.
-
Reward: From Basic Reinforcers to Anticipation of Social Cues.Curr Top Behav Neurosci. 2017;30:207-221. doi: 10.1007/7854_2015_429. Curr Top Behav Neurosci. 2017. PMID: 26728170 Review.
Cited by
-
Naltrexone dose-selectively modulates goal-directed behavior and the hypothalamic proteome in rats.Pharmacol Rep. 2025 Aug;77(4):983-998. doi: 10.1007/s43440-025-00735-4. Epub 2025 May 28. Pharmacol Rep. 2025. PMID: 40434617 Free PMC article.
-
Opioidergic tuning of social attachment: reciprocal relationship between social deprivation and opioid abuse.Front Neuroanat. 2025 Jan 23;18:1521016. doi: 10.3389/fnana.2024.1521016. eCollection 2024. Front Neuroanat. 2025. PMID: 39917739 Free PMC article. Review.
-
Opioidergic modulation of monetary incentive delay fMRI responses.Psychopharmacology (Berl). 2025 Aug;242(8):1743-1756. doi: 10.1007/s00213-025-06753-7. Epub 2025 Feb 12. Psychopharmacology (Berl). 2025. PMID: 40053107 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

