TOP2A modulates signaling via the AKT/mTOR pathway to promote ovarian cancer cell proliferation
- PMID: 38445610
- PMCID: PMC10936659
- DOI: 10.1080/15384047.2024.2325126
TOP2A modulates signaling via the AKT/mTOR pathway to promote ovarian cancer cell proliferation
Abstract
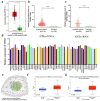
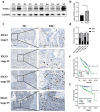
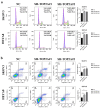
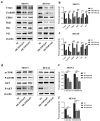
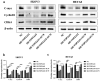
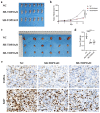
Ovarian cancer (OC) is a form of gynecological malignancy that is associated with worse patient outcomes than any other cancer of the female reproductive tract. Topoisomerase II α (TOP2A) is commonly regarded as an oncogene that is associated with malignant disease progression in a variety of cancers, its mechanistic functions in OC have yet to be firmly established. We explored the role of TOP2A in OC through online databases, clinical samples, in vitro and in vivo experiments. And initial analyses of public databases revealed high OC-related TOP2A expression in patient samples that was related to poorer prognosis. This was confirmed by clinical samples in which TOP2A expression was elevated in OC relative to healthy tissue. Kaplan-Meier analyses further suggested that higher TOP2A expression levels were correlated with worse prognosis in OC patients. In vitro, TOP2A knockdown resulted in the inhibition of OC cell proliferation, with cells entering G1 phase arrest and undergoing consequent apoptotic death. In rescue assays, TOP2A was confirmed to regulate cell proliferation and cell cycle through AKT/mTOR pathway activity. Mouse model experiments further affirmed the key role that TOP2A plays as a driver of OC cell proliferation. These data provide strong evidence supporting TOP2A as an oncogenic mediator and prognostic biomarker related to OC progression and poor outcomes. At the mechanistic level, TOP2A can control tumor cell growth via AKT/mTOR pathway modulation. These preliminary results provide a foundation for future research seeking to explore the utility of TOP2A inhibitor-based combination treatment regimens in platinum-resistant recurrent OC patients.
Keywords: AKT/mTOR signaling pathway; Ovarian cancer; TOP2A; prognosis; proliferation; rescue experiments.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Topoisomerase II alpha promotes gallbladder cancer proliferation and metastasis through activating phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway.Chin Med J (Engl). 2020 Oct 5;133(19):2321-2329. doi: 10.1097/CM9.0000000000001075. Chin Med J (Engl). 2020. PMID: 32925281 Free PMC article.
-
The miRNA mir-582-3p suppresses ovarian cancer progression by targeting AKT/MTOR signaling via lncRNA TUG1.Bioengineered. 2021 Dec;12(2):10771-10781. doi: 10.1080/21655979.2021.2003662. Bioengineered. 2021. PMID: 34793263 Free PMC article.
-
Rhophilin rho GTPase binding protein 1-antisense RNA 1 (RHPN1-AS1) promotes ovarian carcinogenesis by sponging microRNA-485-5p and releasing DNA topoisomerase II alpha (TOP2A).Bioengineered. 2021 Dec;12(2):12003-12022. doi: 10.1080/21655979.2021.2002494. Bioengineered. 2021. PMID: 34787052 Free PMC article.
-
Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance.Semin Cancer Biol. 2019 Dec;59:147-160. doi: 10.1016/j.semcancer.2019.05.012. Epub 2019 May 22. Semin Cancer Biol. 2019. PMID: 31128298 Review.
-
The Role of the Plasminogen Activator Inhibitor 1 ( PAI1 ) in Ovarian Cancer: Mechanisms and Therapeutic Implications.Glob Med Genet. 2024 Oct 29;11(4):358-365. doi: 10.1055/s-0044-1791734. eCollection 2024 Dec. Glob Med Genet. 2024. PMID: 39583124 Free PMC article. Review.
Cited by
-
Exploring the Anti-Leukemic Effect of the Synthetic Retinoid ST1926 on Malignant T Cells: A Comprehensive Proteomics Approach.Int J Mol Sci. 2025 May 13;26(10):4651. doi: 10.3390/ijms26104651. Int J Mol Sci. 2025. PMID: 40429796 Free PMC article.
-
Targeting TOP2A in Ovarian Cancer: Biological and Clinical Implications.Curr Oncol. 2024 Dec 20;31(12):8054-8074. doi: 10.3390/curroncol31120594. Curr Oncol. 2024. PMID: 39727717 Free PMC article. Review.
-
Phillyrin regulates the JAK2/STAT3 signaling pathway by inhibiting TOP2A expression to accelerate ferroptosis in hepatocellular carcinoma.Oncol Rep. 2025 Apr;53(4):43. doi: 10.3892/or.2025.8876. Epub 2025 Feb 14. Oncol Rep. 2025. PMID: 39950325 Free PMC article.
-
Advances in research on malignant tumors and targeted agents for TOP2A (Review).Mol Med Rep. 2025 Feb;31(2):50. doi: 10.3892/mmr.2024.13415. Epub 2024 Dec 13. Mol Med Rep. 2025. PMID: 39670307 Free PMC article. Review.
-
Machine learning based intratumor heterogeneity related signature for prognosis and drug sensitivity in breast cancer.Sci Rep. 2025 Mar 28;15(1):10828. doi: 10.1038/s41598-025-92695-1. Sci Rep. 2025. PMID: 40155597 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
