Factors influencing adverse events following COVID-19 vaccination
- PMID: 38445666
- PMCID: PMC10936640
- DOI: 10.1080/21645515.2024.2323853
Factors influencing adverse events following COVID-19 vaccination
Abstract
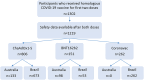
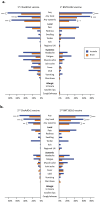
Various novel platform technologies have been used for the development of COVID-19 vaccines. In this nested cohort study among healthcare workers in Australia and Brazil who received three different COVID-19-specific vaccines, we (a) evaluated the incidence of adverse events following immunization (AEFI); (b) compared AEFI by vaccine type, dose and country; (c) identified factors influencing the incidence of AEFI; and (d) assessed the association between reactogenicity and vaccine anti-spike IgG antibody responses. Of 1302 participants who received homologous 2-dose regimens of ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech) or CoronaVac (Sinovac), 1219 (94%) completed vaccine reaction questionnaires. Following the first vaccine dose, the incidence of any systemic reaction was higher in ChAdOx1-S recipients (374/806, 46%) compared with BNT162b2 (55/151, 36%; p = 0.02) or CoronaVac (26/262, 10%; p < 0.001) recipients. After the second vaccine dose, the incidence of any systemic reaction was higher in BNT162b2 recipients (66/151, 44%) compared with ChAdOx1-S (164/806, 20%; p < 0.001) or CoronaVac (23/262, 9%; p < 0.001) recipients. AEFI risk was higher in younger participants, females, participants in Australia, and varied by vaccine type and dose. Prior COVID-19 did not impact the risk of AEFI. Participants in Australia compared with Brazil reported a higher incidence of any local reaction (170/231, 74% vs 222/726, 31%, p < 0.001) and any systemic reaction (171/231, 74% vs 328/726, 45%, p < 0.001), regardless of vaccine type. Following a primary course of ChAdOx1-S or CoronaVac vaccination, participants who did not report AEFI seroconverted at a similar rate to those who reported local or systemic reactions. In conclusion, we found that the incidence of AEFI was influenced by participant age and COVID-19 vaccine type, and differed between participants in Australia and Brazil.
Keywords: COVID-19 vaccine; adverse events; antibody responses.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study.Lancet. 2022 Feb 5;399(10324):521-529. doi: 10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. Lancet. 2022. PMID: 35074136 Free PMC article. Clinical Trial.
-
Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults.Hum Vaccin Immunother. 2022 Nov 30;18(6):2091865. doi: 10.1080/21645515.2022.2091865. Epub 2022 Jul 11. Hum Vaccin Immunother. 2022. PMID: 35816053 Free PMC article. Clinical Trial.
-
Comparison of Adverse Events and Antibody Responses Among Different COVID-19 Vaccination Schedules.Viral Immunol. 2024 Sep;37(7):337-345. doi: 10.1089/vim.2024.0019. Epub 2024 Aug 16. Viral Immunol. 2024. PMID: 39149804
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
-
A scoping review of active, participant centred, digital adverse events following immunization (AEFI) surveillance of WHO approved COVID-19 vaccines: A Canadian immunization Research Network study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2293550. doi: 10.1080/21645515.2023.2293550. Epub 2024 Feb 19. Hum Vaccin Immunother. 2024. PMID: 38374618 Free PMC article.
Cited by
-
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis.Front Public Health. 2024 Jul 30;12:1406315. doi: 10.3389/fpubh.2024.1406315. eCollection 2024. Front Public Health. 2024. PMID: 39139673 Free PMC article.
-
Characteristics of Oral Adverse Effects following COVID-19 Vaccination and Similarities with Oral Symptoms in COVID-19 Patients: Taste and Saliva Secretory Disorders.Med Princ Pract. 2025;34(2):101-120. doi: 10.1159/000543182. Epub 2024 Dec 19. Med Princ Pract. 2025. PMID: 39701050 Free PMC article. Review.
-
Safety and immunogenicity of fractional COVID-19 vaccine doses in Nigerian adults: A randomized non-inferiority trial.Sci Rep. 2025 Jul 29;15(1):27614. doi: 10.1038/s41598-025-06536-2. Sci Rep. 2025. PMID: 40730836 Free PMC article. Clinical Trial.
-
Self-Reported Adverse Events Following COVID-19 Vaccination Among Medical Sciences Students After a Symptomatology Training Program: A Cross-Sectional Study.Health Sci Rep. 2025 Mar 2;8(3):e70492. doi: 10.1002/hsr2.70492. eCollection 2025 Mar. Health Sci Rep. 2025. PMID: 40041786 Free PMC article.
References
-
- World Health Organization . Coronavirus disease (COVID-19) pandemic. 2023. [accessed 2023 May 25]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- World Health Organization . COVID-19 vaccine tracker. 2022. Dec 2 [accessed 2023 Aug 28]. https://covid19.trackvaccines.org/agency/who/.
-
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, Dattani S, Beltekian D, O-O E. et al. Our world in data: coronavirus (COVID-19) vaccinations. 2023. [accessed 2023 Aug 28]. https://ourworldindata.org/coronavirus.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
