Factors influencing adverse events following COVID-19 vaccination
- PMID: 38445666
- PMCID: PMC10936640
- DOI: 10.1080/21645515.2024.2323853
Factors influencing adverse events following COVID-19 vaccination
Abstract
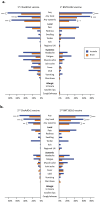
Various novel platform technologies have been used for the development of COVID-19 vaccines. In this nested cohort study among healthcare workers in Australia and Brazil who received three different COVID-19-specific vaccines, we (a) evaluated the incidence of adverse events following immunization (AEFI); (b) compared AEFI by vaccine type, dose and country; (c) identified factors influencing the incidence of AEFI; and (d) assessed the association between reactogenicity and vaccine anti-spike IgG antibody responses. Of 1302 participants who received homologous 2-dose regimens of ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech) or CoronaVac (Sinovac), 1219 (94%) completed vaccine reaction questionnaires. Following the first vaccine dose, the incidence of any systemic reaction was higher in ChAdOx1-S recipients (374/806, 46%) compared with BNT162b2 (55/151, 36%; p = 0.02) or CoronaVac (26/262, 10%; p < 0.001) recipients. After the second vaccine dose, the incidence of any systemic reaction was higher in BNT162b2 recipients (66/151, 44%) compared with ChAdOx1-S (164/806, 20%; p < 0.001) or CoronaVac (23/262, 9%; p < 0.001) recipients. AEFI risk was higher in younger participants, females, participants in Australia, and varied by vaccine type and dose. Prior COVID-19 did not impact the risk of AEFI. Participants in Australia compared with Brazil reported a higher incidence of any local reaction (170/231, 74% vs 222/726, 31%, p < 0.001) and any systemic reaction (171/231, 74% vs 328/726, 45%, p < 0.001), regardless of vaccine type. Following a primary course of ChAdOx1-S or CoronaVac vaccination, participants who did not report AEFI seroconverted at a similar rate to those who reported local or systemic reactions. In conclusion, we found that the incidence of AEFI was influenced by participant age and COVID-19 vaccine type, and differed between participants in Australia and Brazil.
Keywords: COVID-19 vaccine; adverse events; antibody responses.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- World Health Organization . Coronavirus disease (COVID-19) pandemic. 2023. [accessed 2023 May 25]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- World Health Organization . COVID-19 vaccine tracker. 2022. Dec 2 [accessed 2023 Aug 28]. https://covid19.trackvaccines.org/agency/who/.
-
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, Dattani S, Beltekian D, O-O E. et al. Our world in data: coronavirus (COVID-19) vaccinations. 2023. [accessed 2023 Aug 28]. https://ourworldindata.org/coronavirus.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
