Molecular mechanisms that govern infection and antifungal resistance in Mucorales
- PMID: 38445820
- PMCID: PMC10966947
- DOI: 10.1128/mmbr.00188-22
Molecular mechanisms that govern infection and antifungal resistance in Mucorales
Abstract
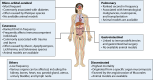
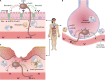
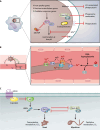
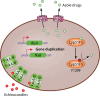
SUMMARYThe World Health Organization has established a fungal priority pathogens list that includes species critical or highly important to human health. Among them is the order Mucorales, a fungal group comprising at least 39 species responsible for the life-threatening infection known as mucormycosis. Despite the continuous rise in cases and the poor prognosis due to innate resistance to most antifungal drugs used in the clinic, Mucorales has received limited attention, partly because of the difficulties in performing genetic manipulations. The COVID-19 pandemic has further escalated cases, with some patients experiencing the COVID-19-associated mucormycosis, highlighting the urgent need to increase knowledge about these fungi. This review addresses significant challenges in treating the disease, including delayed and poor diagnosis, the lack of accurate global incidence estimation, and the limited treatment options. Furthermore, it focuses on the most recent discoveries regarding the mechanisms and genes involved in the development of the disease, antifungal resistance, and the host defense response. Substantial advancements have been made in identifying key fungal genes responsible for invasion and tissue damage, host receptors exploited by the fungus to invade tissues, and mechanisms of antifungal resistance. This knowledge is expected to pave the way for the development of new antifungals to combat mucormycosis. In addition, we anticipate significant progress in characterizing Mucorales biology, particularly the mechanisms involved in pathogenesis and antifungal resistance, with the possibilities offered by CRISPR-Cas9 technology for genetic manipulation of the previously intractable Mucorales species.
Keywords: CotH; Mucor; RNAi; Rhizopus; dimorphism; genome duplication; host responses; invasins; iron uptake; mucoricin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, et al. . 2019. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis 19:e405–e421. doi:10.1016/S1473-3099(19)30312-3 - DOI - PMC - PubMed
-
- WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization (WHO). Available from: https://www.who.int/publications/ i/item/9789240060241
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

