The microbiome dynamics and interaction of endosymbiotic Symbiodiniaceae and fungi are associated with thermal bleaching susceptibility of coral holobionts
- PMID: 38445866
- PMCID: PMC11022545
- DOI: 10.1128/aem.01939-23
The microbiome dynamics and interaction of endosymbiotic Symbiodiniaceae and fungi are associated with thermal bleaching susceptibility of coral holobionts
Abstract
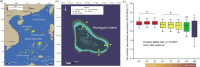
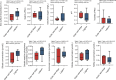
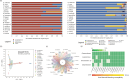
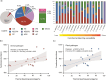
The thermal bleaching percentage of coral holobionts shows interspecific differences under heat-stress conditions, which are closely related to the coral-associated microbiome. However, the ecological effects of community dynamics and interactions between Symbiodiniaceae and fungi on coral thermal bleaching susceptibility remain unclear. In this study, we analyzed the diversity, community structure, functions, and potential interaction of Symbiodiniaceae and fungi among 18 coral species from a high thermal bleaching risk atoll using next-generation sequencing. The results showed that heat-tolerant C3u sub-clade and Durusdinium dominated the Symbiodiniaceae community of corals and that there were no core amplicon sequence variants in the coral-associated fungal community. Fungal richness and the abundance of confirmed functional animal-plant pathogens were significantly positively correlated with the coral thermal bleaching percentage. Fungal indicators, including Didymellaceae, Chaetomiaceae, Schizophyllum, and Colletotrichum, were identified in corals. Each coral species had a complex Symbiodiniaceae-fungi interaction network (SFIN), which was driven by the dominant Symbiodiniaceae sub-clades. The SFINs of coral holobionts with low thermal bleaching susceptibility exhibited low complexity and high betweenness centrality. These results indicate that the extra heat tolerance of coral in Huangyan Island may be linked to the high abundance of heat-tolerant Symbiodiniaceae. Fungal communities have high interspecific flexibility, and the increase of fungal diversity and pathogen abundance was correlated with higher thermal bleaching susceptibility of corals. Moreover, fungal indicators were associated with the degrees of coral thermal bleaching susceptibility, including both high and intermediate levels. The topological properties of SFINs suggest that heat-tolerant coral have limited fungal parasitism and strong microbial network resilience.IMPORTANCEGlobal warming and enhanced marine heatwaves have led to a rapid decline in coral reef ecosystems worldwide. Several studies have focused on the impact of coral-associated microbiomes on thermal bleaching susceptibility in corals; however, the ecological functions and interactions between Symbiodiniaceae and fungi remain unclear. We investigated the microbiome dynamics and potential interactions of Symbiodiniaceae and fungi among 18 coral species in Huangyan Island. Our study found that the Symbiodiniaceae community of corals was mainly composed of heat-tolerant C3u sub-clade and Durusdinium. The increase in fungal diversity and pathogen abundance has close associations with higher coral thermal bleaching susceptibility. We first constructed an interaction network between Symbiodiniaceae and fungi in corals, which indicated that restricting fungal parasitism and strong interaction network resilience would promote heat acclimatization of corals. Accordingly, this study provides insights into the role of microorganisms and their interaction as drivers of interspecific differences in coral thermal bleaching.
Keywords: Symbiodiniaceae; coral holobiont; fungi; microbiome dynamics; potential interaction; thermal bleaching susceptibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Evolutionary radiation and microbial community dynamics shape the thermal tolerance of Fungiidae in the southern South China Sea.Microbiol Spectr. 2024 Feb 6;12(2):e0243623. doi: 10.1128/spectrum.02436-23. Epub 2024 Jan 4. Microbiol Spectr. 2024. PMID: 38174936 Free PMC article.
-
Microbiome community and complexity indicate environmental gradient acclimatisation and potential microbial interaction of endemic coral holobionts in the South China Sea.Sci Total Environ. 2021 Apr 15;765:142690. doi: 10.1016/j.scitotenv.2020.142690. Epub 2020 Oct 3. Sci Total Environ. 2021. PMID: 33071127
-
Hawaiian coral holobionts reveal algal and prokaryotic host specificity, intraspecific variability in bleaching resistance, and common interspecific microbial consortia modulating thermal stress responses.Sci Total Environ. 2023 Sep 1;889:164040. doi: 10.1016/j.scitotenv.2023.164040. Epub 2023 May 18. Sci Total Environ. 2023. PMID: 37209745
-
Coral evolutionary responses to microbial symbioses.Philos Trans R Soc Lond B Biol Sci. 2020 Sep 28;375(1808):20190591. doi: 10.1098/rstb.2019.0591. Epub 2020 Aug 10. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32772672 Free PMC article. Review.
-
Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks.Environ Microbiol. 2020 May;22(5):1675-1687. doi: 10.1111/1462-2920.14918. Epub 2020 Jan 23. Environ Microbiol. 2020. PMID: 31943674 Review.
Cited by
-
Beneficial and detrimental fungi within the culturable mycobiome of the Red Sea coral Stylophora pistilatta.ISME J. 2025 Jan 2;19(1):wraf090. doi: 10.1093/ismejo/wraf090. ISME J. 2025. PMID: 40318223 Free PMC article.
-
Symbiotic Symbiodiniaceae mediate coral-associated bacterial communities along a natural thermal gradient.Environ Microbiome. 2025 Jun 17;20(1):72. doi: 10.1186/s40793-025-00733-2. Environ Microbiome. 2025. PMID: 40528245 Free PMC article.
References
-
- Voolstra CR, Suggett DJ, Peixoto RS, Parkinson JE, Quigley KM, Silveira CB, Sweet M, Muller EM, Barshis DJ, Bourne DG, Aranda M. 2021. Extending the natural adaptive capacity of coral holobionts. Nat Rev Earth Environ 2:747–762. doi:10.1038/s43017-021-00214-3 - DOI
Publication types
MeSH terms
Grants and funding
- 42090041, 42030502/MOST | National Natural Science Foundation of China (NSFC)
- 42306165/MOST | National Natural Science Foundation of China (NSFC)
- GXLSCRSCS2021103/Self-Topic Project of Guangxi Laboratory on the Study of Coral Reefs in the South China Sea
- PM-zx703-202105-176, PM-zx703-202004-143/Central Public-Interest Scientific Institution Basal Fund
LinkOut - more resources
Full Text Sources

