Rickettsia rickettsii virulence determinants RARP2 and RapL mitigate IFN- β signaling in primary human dermal microvascular endothelial cells
- PMID: 38445878
- PMCID: PMC11005427
- DOI: 10.1128/mbio.03450-23
Rickettsia rickettsii virulence determinants RARP2 and RapL mitigate IFN- β signaling in primary human dermal microvascular endothelial cells
Abstract
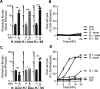
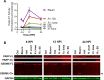
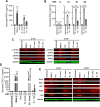
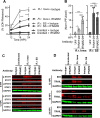
We compared the growth characteristics of a virulent Rickettsia rickettsii strain (Sheila Smith) to an attenuated R. rickettsii stain (Iowa) and a non-pathogenic species (R. montanensis) in primary human dermal microvascular endothelial cells (HDMEC). All replicated in Vero cells, however, only the Sheila Smith strain productively replicated in HDMECs. The Iowa strain showed minimal replication over a 24-h period, while R. montanensis lost viability and induced lysis of the HDMECs via a rapid programmed cell death response. Both the virulent and attenuated R. rickettsii strains, but not R. montanensis, induced an interferon-1 response, although the response was of lesser magnitude and delayed in the Sheila Smith strain. IFN-β secretion correlated with increased host cell lysis, and treatment with anti-IFNAR2 antibody decreased lysis from Iowa-infected but not Sheila Smith-infected cells. Both Sheila Smith- and Iowa-infected cells eventually lysed, although the response from Sheila Smith was delayed and showed characteristics of apoptosis. We, therefore, examined whether reconstitution of the Iowa strain with two recently described putative virulence determinants might enhance survival of Iowa within HDMECs. Reconstitution with RARP2, which is inhibitory to anterograde trafficking through the Golgi apparatus, reduced IFN-β secretion but had no effect on cell lysis. RapL, which proteolytically processes surface exposed autotransporters and enhances replication of Iowa in Guinea pigs, suppressed both IFN-β production and host cell lysis. These findings suggest distinct mechanisms by which virulent spotted fever group rickettsiae may enhance intracellular survival and replication.IMPORTANCEWe examined a naturally occurring non-pathogenic rickettsial species, R. montanensis, a laboratory-attenuated R. rickettsii strain (Iowa), and a fully virulent R. rickettsii strain (Sheila Smith) for growth in human dermal microvascular endothelial cells. The two avirulent strains replicated poorly or not at all. Only the virulent Sheila Smith strain replicated. IFN-β production correlated with the inhibition of R. rickettsii Iowa. Reconstitution of Iowa with either of two recently described putative virulence determinants altered the IFN-β response. A rickettsial ankyrin repeat protein, RARP2, disrupts the trans-Golgi network and inhibits IFN-β secretion. An autotransporter peptidase, RapL, restores proteolytic maturation of outer membrane autotransporters and diminishes the IFN-β response to enhance cell survival and permit replication of the recombinant strain. These studies point the way toward discovery of mechanisms for innate immune response avoidance by virulent rickettsia.
Keywords: Rickettsia; innate immunity; interferon-beta; primary cells; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa.Infect Immun. 2008 Feb;76(2):542-50. doi: 10.1128/IAI.00952-07. Epub 2007 Nov 19. Infect Immun. 2008. PMID: 18025092 Free PMC article.
-
The Rickettsial Ankyrin Repeat Protein 2 Is a Type IV Secreted Effector That Associates with the Endoplasmic Reticulum.mBio. 2018 Jun 26;9(3):e00975-18. doi: 10.1128/mBio.00975-18. mBio. 2018. PMID: 29946049 Free PMC article.
-
Targeted knockout of the Rickettsia rickettsii OmpA surface antigen does not diminish virulence in a mammalian model system.mBio. 2015 Mar 31;6(2):e00323-15. doi: 10.1128/mBio.00323-15. mBio. 2015. PMID: 25827414 Free PMC article.
-
Comparative genome sequencing of Rickettsia rickettsii strains that differ in virulence.Infect Immun. 2015 Apr;83(4):1568-76. doi: 10.1128/IAI.03140-14. Epub 2015 Feb 2. Infect Immun. 2015. PMID: 25644009 Free PMC article.
-
The realities of biodefense vaccines against Rickettsia.Vaccine. 2009 Nov 5;27 Suppl 4(Suppl 4):D52-5. doi: 10.1016/j.vaccine.2009.07.045. Vaccine. 2009. PMID: 19837287 Free PMC article. Review.
Cited by
-
Genomic characterization of an emerging Rickettsia barbariae isolated from tick eggs in northwestern China.Emerg Microbes Infect. 2024 Dec;13(1):2396870. doi: 10.1080/22221751.2024.2396870. Epub 2024 Sep 5. Emerg Microbes Infect. 2024. PMID: 39193640 Free PMC article.
-
The dual role of type I interferons in bacterial infections: from immune defense to pathogenesis.mBio. 2025 Jul 9;16(7):e0148125. doi: 10.1128/mbio.01481-25. Epub 2025 Jun 17. mBio. 2025. PMID: 40525851 Free PMC article. Review.
-
Polysaccharide synthesis operon modulates Rickettsia-endothelial cell interactions.PLoS Pathog. 2025 Jun 26;21(6):e1013277. doi: 10.1371/journal.ppat.1013277. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40570043 Free PMC article.
-
Rickettsia rickettsii RoaM negatively regulates expression of a limited number of rickettsial genes.mSphere. 2025 Apr 29;10(4):e0007725. doi: 10.1128/msphere.00077-25. Epub 2025 Apr 8. mSphere. 2025. PMID: 40197162 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

