Hallmark Molecular and Pathological Features of POLG Disease are Recapitulated in Cerebral Organoids
- PMID: 38445970
- PMCID: PMC11095234
- DOI: 10.1002/advs.202307136
Hallmark Molecular and Pathological Features of POLG Disease are Recapitulated in Cerebral Organoids
Abstract
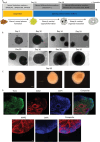
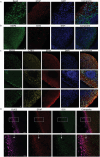
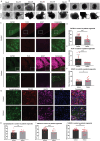
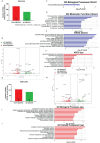
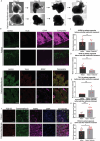
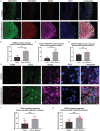
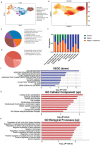
In this research, a 3D brain organoid model is developed to study POLG-related encephalopathy, a mitochondrial disease stemming from POLG mutations. Induced pluripotent stem cells (iPSCs) derived from patients with these mutations is utilized to generate cortical organoids, which exhibited typical features of the diseases with POLG mutations, such as altered morphology, neuronal loss, and mitochondiral DNA (mtDNA) depletion. Significant dysregulation is also identified in pathways crucial for neuronal development and function, alongside upregulated NOTCH and JAK-STAT signaling pathways. Metformin treatment ameliorated many of these abnormalities, except for the persistent affliction of inhibitory dopamine-glutamate (DA GLU) neurons. This novel model effectively mirrors both the molecular and pathological attributes of diseases with POLG mutations, providing a valuable tool for mechanistic understanding and therapeutic screening for POLG-related disorders and other conditions characterized by compromised neuronal mtDNA maintenance and complex I deficiency.
Keywords: POLG; cortical organoids; iPSC; mitochondrial function; neuron.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Activation of Neurotoxic Astrocytes Due to Mitochondrial Dysfunction Triggered by POLG Mutation.Int J Biol Sci. 2024 May 11;20(8):2860-2880. doi: 10.7150/ijbs.93445. eCollection 2024. Int J Biol Sci. 2024. PMID: 38904024 Free PMC article.
-
Deoxyribonucleoside treatment rescues EtBr-induced mtDNA depletion in iPSC-derived neural stem cells with POLG mutations.FASEB J. 2023 Sep;37(9):e23139. doi: 10.1096/fj.202300650RR. FASEB J. 2023. PMID: 37584631
-
The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC derived Cortical Organoid of Alpers' Disease.Int J Biol Sci. 2024 Jan 25;20(4):1194-1217. doi: 10.7150/ijbs.91624. eCollection 2024. Int J Biol Sci. 2024. PMID: 38385069 Free PMC article.
-
POLG-related disorders and their neurological manifestations.Nat Rev Neurol. 2019 Jan;15(1):40-52. doi: 10.1038/s41582-018-0101-0. Nat Rev Neurol. 2019. PMID: 30451971 Free PMC article. Review.
-
Modeling mitochondrial DNA diseases: from base editing to pluripotent stem-cell-derived organoids.EMBO Rep. 2023 Apr 5;24(4):e55678. doi: 10.15252/embr.202255678. Epub 2023 Mar 6. EMBO Rep. 2023. PMID: 36876467 Free PMC article. Review.
Cited by
-
Cannabinerol Restores mRNA Splicing Defects Induced by β-Amyloid in an In Vitro Model of Alzheimer's Disease: A Transcriptomic Study.Int J Mol Sci. 2025 Mar 28;26(7):3113. doi: 10.3390/ijms26073113. Int J Mol Sci. 2025. PMID: 40243843 Free PMC article.
-
Biomedical applications of organoids in genetic diseases.Med Rev (2021). 2024 Dec 24;5(2):152-163. doi: 10.1515/mr-2024-0077. eCollection 2025 Apr. Med Rev (2021). 2024. PMID: 40224362 Free PMC article. Review.
References
-
- Lamantea E., Tiranti V., Bordoni A., Toscano A., Bono F., Servidei S., Papadimitriou A., Spelbrink H., Silvestri L., Casari G., Comi G. P., Zeviani M., Ann. Neurol. 2002, 52, 211. - PubMed
-
- Van Goethem G., Martin J. J., Dermaut B., Löfgren A., Wibail A., Ververken D., Tack P., Dehaene I., Van Zandijcke M., Moonen M., Ceuterick C., De Jonghe P., Van Broeckhoven C., Neuromuscular Disord. 2003, 13, 133. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
