Semi-supervised Learning for Generalizable Intracranial Hemorrhage Detection and Segmentation
- PMID: 38446043
- PMCID: PMC11140498
- DOI: 10.1148/ryai.230077
Semi-supervised Learning for Generalizable Intracranial Hemorrhage Detection and Segmentation
Abstract
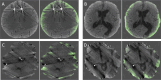
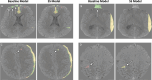
Purpose To develop and evaluate a semi-supervised learning model for intracranial hemorrhage detection and segmentation on an out-of-distribution head CT evaluation set. Materials and Methods This retrospective study used semi-supervised learning to bootstrap performance. An initial "teacher" deep learning model was trained on 457 pixel-labeled head CT scans collected from one U.S. institution from 2010 to 2017 and used to generate pseudo labels on a separate unlabeled corpus of 25 000 examinations from the Radiological Society of North America and American Society of Neuroradiology. A second "student" model was trained on this combined pixel- and pseudo-labeled dataset. Hyperparameter tuning was performed on a validation set of 93 scans. Testing for both classification (n = 481 examinations) and segmentation (n = 23 examinations, or 529 images) was performed on CQ500, a dataset of 481 scans performed in India, to evaluate out-of-distribution generalizability. The semi-supervised model was compared with a baseline model trained on only labeled data using area under the receiver operating characteristic curve, Dice similarity coefficient, and average precision metrics. Results The semi-supervised model achieved a statistically significant higher examination area under the receiver operating characteristic curve on CQ500 compared with the baseline (0.939 [95% CI: 0.938, 0.940] vs 0.907 [95% CI: 0.906, 0.908]; P = .009). It also achieved a higher Dice similarity coefficient (0.829 [95% CI: 0.825, 0.833] vs 0.809 [95% CI: 0.803, 0.812]; P = .012) and pixel average precision (0.848 [95% CI: 0.843, 0.853]) vs 0.828 [95% CI: 0.817, 0.828]) compared with the baseline. Conclusion The addition of unlabeled data in a semi-supervised learning framework demonstrates stronger generalizability potential for intracranial hemorrhage detection and segmentation compared with a supervised baseline. Keywords: Semi-supervised Learning, Traumatic Brain Injury, CT, Machine Learning Supplemental material is available for this article. Published under a CC BY 4.0 license. See also the commentary by Swimburne in this issue.
Keywords: CT; Machine Learning; Semi-supervised Learning; Traumatic Brain Injury.
Conflict of interest statement
Figures


Comment in
-
When the Student Becomes the Master: Boosting Intracranial Hemorrhage Detection Generalizability with Teacher-Student Learning.Radiol Artif Intell. 2024 May;6(3):e240126. doi: 10.1148/ryai.240126. Radiol Artif Intell. 2024. PMID: 38597790 Free PMC article. No abstract available.
References
-
- Titano JJ , Badgeley M , Schefflein J , et al. . Automated deep-neural-network surveillance of cranial images for acute neurologic events . Nat Med 2018. ; 24 ( 9 ): 1337 – 1341 . - PubMed
-
- Chilamkurthy S , Ghosh R , Tanamala S , et al. . Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study . Lancet 2018. ; 392 ( 10162 ): 2388 – 2396 . - PubMed
-
- Prevedello LM , Erdal BS , Ryu JL , et al. . Automated critical test findings identification and online notification system using artificial intelligence in imaging . Radiology 2017. ; 285 ( 3 ): 923 – 931 . - PubMed
-
- Lee H , Yune S , Mansouri M , et al. . An explainable deep-learning algorithm for the detection of acute intracranial haemorrhage from small datasets . Nat Biomed Eng 2019. ; 3 ( 3 ): 173 – 182 . - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

