Ureidopenicillins Are Potent Inhibitors of Penicillin-Binding Protein 2 from Multidrug-Resistant Neisseria gonorrhoeae H041
- PMID: 38446051
- PMCID: PMC11812267
- DOI: 10.1021/acsinfecdis.3c00713
Ureidopenicillins Are Potent Inhibitors of Penicillin-Binding Protein 2 from Multidrug-Resistant Neisseria gonorrhoeae H041
Abstract
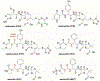
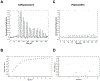
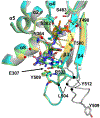
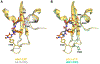
Effective treatment of gonorrhea is threatened by the increasing prevalence of Neisseria gonorrhoeae strains resistant to the extended-spectrum cephalosporins (ESCs). Recently, we demonstrated the promise of the third-generation cephalosporin cefoperazone as an antigonococcal agent due to its rapid second-order rate of acylation against penicillin-binding protein 2 (PBP2) from the ESC-resistant strain H041 and robust antimicrobial activity against H041. Noting the presence of a ureido moiety in cefoperazone, we evaluated a subset of structurally similar ureido β-lactams, including piperacillin, azlocillin, and mezlocillin, for activity against PBP2 from H041 using biochemical and structural analyses. We found that the ureidopenicillin piperacillin has a second-order rate of acylation against PBP2 that is 12-fold higher than cefoperazone and 85-fold higher than ceftriaxone and a lower MIC against H041 than ceftriaxone. Surprisingly, the affinity of ureidopenicillins for PBP2 is minimal, indicating that their inhibitory potency is due to a higher rate of the acylation step of the reaction compared to cephalosporins. Enhanced acylation results from the combination of a penam scaffold with a 2,3-dioxopiperazine-containing R1 group. Crystal structures show that the ureido β-lactams overcome the effects of resistance mutations present in PBP2 from H041 by eliciting conformational changes that are hindered when PBP2 interacts with the weaker inhibitor ceftriaxone. Overall, our results support the potential of piperacillin as a treatment for gonorrhea and provide a framework for the future design of β-lactams with improved activity against ESC-resistant N. gonorrhoeae.
Keywords: Neisseria gonorrhoeae; antimicrobial resistance; penicillin-binding protein 2; β-lactams.
Figures


Similar articles
-
Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae.ACS Infect Dis. 2021 Feb 12;7(2):293-308. doi: 10.1021/acsinfecdis.0c00400. Epub 2021 Feb 3. ACS Infect Dis. 2021. PMID: 33533239 Free PMC article.
-
Affinity of β-Lactam Antibiotics for Neisseria gonorrhoeae Penicillin-Binding Protein 2 Having Wild, Cefixime-Reduced-Susceptible, and Cephalosporin (Ceftriaxone)-Resistant penA Alleles.Microb Drug Resist. 2024 Mar;30(3):141-146. doi: 10.1089/mdr.2023.0256. Epub 2024 Jan 12. Microb Drug Resist. 2024. PMID: 38215246
-
Mutations in penicillin-binding protein 2 from cephalosporin-resistant Neisseria gonorrhoeae hinder ceftriaxone acylation by restricting protein dynamics.J Biol Chem. 2020 May 22;295(21):7529-7543. doi: 10.1074/jbc.RA120.012617. Epub 2020 Apr 6. J Biol Chem. 2020. PMID: 32253235 Free PMC article.
-
Global resistance of Neisseria gonorrhoeae: when theory becomes reality.Curr Opin Infect Dis. 2014 Feb;27(1):62-7. doi: 10.1097/QCO.0000000000000025. Curr Opin Infect Dis. 2014. PMID: 24275696 Review.
-
Multiresistant Neisseria gonorrhoeae: a new threat in second decade of the XXI century.Med Microbiol Immunol. 2020 Apr;209(2):95-108. doi: 10.1007/s00430-019-00651-4. Epub 2019 Dec 4. Med Microbiol Immunol. 2020. PMID: 31802195 Free PMC article. Review.
Cited by
-
Differential contribution of PBP occupancy and efflux on the effectiveness of β-lactams at their target site in clinical isolates of Neisseria gonorrhoeae.PLoS Pathog. 2024 Dec 31;20(12):e1012783. doi: 10.1371/journal.ppat.1012783. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39739989 Free PMC article.
-
A new class of penicillin-binding protein inhibitors to address drug-resistant Neisseria gonorrhoeae.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.630553. doi: 10.1101/2024.12.27.630553. bioRxiv. 2024. PMID: 39763734 Free PMC article. Preprint.
References
-
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2021. 2021. (accessed 05/19/2023).
-
- World Health Organisation. Report on global sexually transmitted infection surveillance 2018. World Health Organization: 2018.
-
- Cohen MS; Hoffman IF; Royce RA; Kazembe P; Dyer JR; Daly CC; Zimba D; Vernazza PL; Maida M; Fiscus SA; Eron JJ Jr. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997, 349, 1868–1873. DOI: 10.1016/s0140-6736(97)02190-9. - DOI - PubMed
-
- Ghys PD; Fransen K; Diallo MO; Ettiegne-Traore V; Coulibaly IM; Yeboue KM; Kalish ML; Maurice C; Whitaker JP; Greenberg AE; Laga M The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d’Ivoire. AIDS 1997, 11, F85–93. DOI: 10.1097/00002030-199712000-00001. - DOI - PubMed
-
- Shafer WM; Folster JP; Nicholas RA Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseriae. In Neisseria: Molecular Mechanisms of Pathogenesis, Genco CA, L. WEds.; Horizon Scientific Press, 2010; p 245.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

