Regulation of phase separation and antiviral activity of Cactin by glycolytic enzyme PGK via phosphorylation in Drosophila
- PMID: 38446061
- PMCID: PMC11005415
- DOI: 10.1128/mbio.01378-23
Regulation of phase separation and antiviral activity of Cactin by glycolytic enzyme PGK via phosphorylation in Drosophila
Abstract
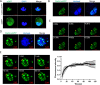
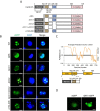
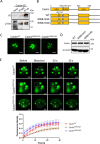
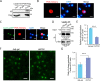
Liquid-liquid phase separation (LLPS) plays a crucial role in various biological processes in eukaryotic organisms, including immune responses in mammals. However, the specific function of LLPS in immune responses in Drosophila melanogaster remains poorly understood. Cactin, a highly conserved protein in eukaryotes, is involved in a non-canonical signaling pathway associated with Nuclear factor-κB (NF-κB)-related pathways in Drosophila. In this study, we investigated the role of Cactin in LLPS and its implications for immune response modulation. We discovered that Cactin undergoes LLPS, forming droplet-like particles, primarily mediated by its intrinsically disordered region (IDR). Utilizing immunoprecipitation and mass spectrometry analysis, we identified two phosphorylation sites at serine residues 99 and 104 within the IDR1 domain of Cactin. Co-immunoprecipitation and mass spectrometry further revealed phosphoglycerate kinase (PGK) as a Cactin-interacting protein responsible for regulating its phosphorylation. Phosphorylation of Cactin by PGK induced a transition from stable aggregates to dynamic liquid droplets, enhancing its ability to interact with other components in the cellular environment. Overexpression of PGK inhibited Drosophila C virus (DCV) replication, while PGK knockdown increased replication. DCV infection also increased Cactin phosphorylation. We also found that phosphorylation enhances the antiviral ability of Cactin by promoting liquid-phase droplet formation. These findings demonstrate the role of Cactin-phase separation in regulating DCV replication and highlight the modulation of its antiviral function through phosphorylation, providing insights into the interplay between LLPS and antiviral defense mechanisms.
Importance: Liquid-liquid phase separation (LLPS) plays an integral role in various biological processes in eukaryotic organisms. Although several studies have highlighted its crucial role in modulating immune responses in mammals, its function in immune responses in Drosophila melanogaster remains poorly understood. Our study investigated the role of Cactin in LLPS and its implications for immune response modulation. We identified that phosphoglycerate kinase (PGK), an essential enzyme in the glycolytic pathway, phosphorylates Cactin, facilitating its transition from a relatively stable aggregated state to a more dynamic liquid droplet phase during the phase separation process. This transformation allows Cactin to rapidly interact with other cellular components, enhancing its antiviral properties and ultimately inhibiting virus replication. These findings expand our understanding of the role of LLPS in the antiviral defense mechanism, shedding light on the intricate mechanisms underlying immune responses in D. melanogaster.
Keywords: Cactin; Drosophila C virus; Drosophila melanogaster; antiviral immunity; liquid–liquid phase separation; phosphoglycerate kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of ubiquitination and antiviral activity of Cactin by deubiquitinase Usp14 in Drosophila.J Virol. 2024 May 14;98(5):e0017724. doi: 10.1128/jvi.00177-24. Epub 2024 Apr 2. J Virol. 2024. PMID: 38563731 Free PMC article.
-
Phosphorylation, disorder, and phase separation govern the behavior of Frequency in the fungal circadian clock.Elife. 2024 Mar 25;12:RP90259. doi: 10.7554/eLife.90259. Elife. 2024. PMID: 38526948 Free PMC article.
-
lncRNA Sensing of a Viral Suppressor of RNAi Activates Non-canonical Innate Immune Signaling in Drosophila.Cell Host Microbe. 2020 Jan 8;27(1):115-128.e8. doi: 10.1016/j.chom.2019.12.006. Cell Host Microbe. 2020. PMID: 31917956
-
[Latest Findings on the Role of Liquid-Liquid Phase Separation in the Regulation of Immune Cell Activation and Key Signaling].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Nov 20;55(6):1527-1532. doi: 10.12182/20241160302. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39990825 Free PMC article. Review. Chinese.
-
Molecular mechanisms and cellular functions of liquid-liquid phase separation during antiviral immune responses.Front Immunol. 2023 May 10;14:1162211. doi: 10.3389/fimmu.2023.1162211. eCollection 2023. Front Immunol. 2023. PMID: 37251408 Free PMC article. Review.
Cited by
-
A manipulating pair.Nat Rev Microbiol. 2024 Apr;22(4):188. doi: 10.1038/s41579-024-01021-5. Nat Rev Microbiol. 2024. PMID: 38347126 No abstract available.
-
Effect of potato glycoside alkaloids on mitochondria energy metabolism of Fusarium solani, the root rot pathogen of Lycium barbarum.Front Microbiol. 2025 Jan 29;15:1512027. doi: 10.3389/fmicb.2024.1512027. eCollection 2024. Front Microbiol. 2025. PMID: 39943965 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
