Ergothioneine promotes longevity and healthy aging in male mice
- PMID: 38446314
- PMCID: PMC11226696
- DOI: 10.1007/s11357-024-01111-5
Ergothioneine promotes longevity and healthy aging in male mice
Abstract
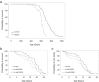
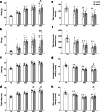
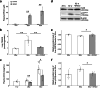
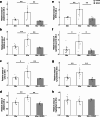
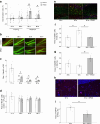
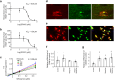
Healthy aging has emerged as a crucial issue with the increase in the geriatric population worldwide. Food-derived sulfur-containing amino acid ergothioneine (ERGO) is a potential dietary supplement, which exhibits various beneficial effects in experimental animals although the preventive effects of ERGO on aging and/or age-related impairments such as frailty and cognitive impairment are unclear. We investigated the effects of daily oral supplementation of ERGO dissolved in drinking water on lifespan, frailty, and cognitive impairment in male mice from 7 weeks of age to the end of their lives. Ingestion of 4 ~ 5 mg/kg/day of ERGO remarkably extended the lifespan of male mice. The longevity effect of ERGO was further supported by increase in life and non-frailty spans of Caenorhabditis elegans in the presence of ERGO. Compared with the control group, the ERGO group showed significantly lower age-related declines in weight, fat mass, and average and maximum movement velocities at 88 weeks of age. This was compatible with dramatical suppression by ERGO of the age-related increments in plasma biomarkers (BMs) such as the chemokine ligand 9, creatinine, symmetric dimethylarginine, urea, asymmetric dimethylarginine, quinolinic acid, and kynurenine. The oral intake of ERGO also rescued age-related impairments in learning and memory ability, which might be associated with suppression of the age-related decline in hippocampal neurogenesis and TDP43 protein aggregation and promotion of microglial shift to the M2 phenotype by ERGO ingestion. Ingestion of ERGO may promote longevity and healthy aging in male mice, possibly through multiple biological mechanisms.
Keywords: Age-related impairments; Anti-aging; Ergothioneine; Frailty; Healthy aging; Hippocampal neurogenesis; Lifespan; Longevity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Sayed N, HuangY, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T, Tibshirani R, Hastie T, Alpert A, Cui L, Kuznetsova T, Rosenberg-Hasson Y, Ostan R, Monti D, Lehallier B, Shen-Orr SS, Maecker HT, Dekker CL, Wyss-Coray T, … Furman D. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity immunosenescence frailty and cardiovascular aging. Nat Aging 2021 1:598–615. 10.1038/s43587-021-00082-y - PMC - PubMed
-
- Heneka MT, Carson MJ, Khoury JEL Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, … Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015,14(4):388–405.10.1016/S1474-4422(15)70016-5. - PMC - PubMed
-
- Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA, Loane DJ. Old age increases microglial senescence exacerbates secondary neuroinflammation and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol Aging. 2019;77:194–206. doi: 10.1016/j.neurobiolaging.2019.02.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

