Metabolism of the 4-Hydroxyphenylpyruvate Dioxygenase Inhibitor, Mesotrione, in Multiple-Herbicide-Resistant Palmer amaranth (Amaranthus palmeri)
- PMID: 38446412
- PMCID: PMC10959109
- DOI: 10.1021/acs.jafc.3c06903
Metabolism of the 4-Hydroxyphenylpyruvate Dioxygenase Inhibitor, Mesotrione, in Multiple-Herbicide-Resistant Palmer amaranth (Amaranthus palmeri)
Abstract
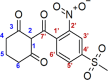
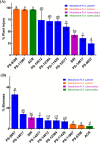
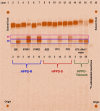
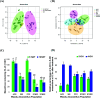
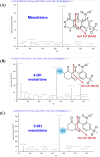
Metabolic resistance to the maize-selective, HPPD-inhibiting herbicide, mesotrione, occurs via Phase I ring hydroxylation in resistant waterhemp and Palmer amaranth; however, mesotrione detoxification pathways post-Phase I are unknown. This research aims to (1) evaluate Palmer amaranth populations for mesotrione resistance via survivorship, foliar injury, and aboveground biomass, (2) determine mesotrione metabolism rates in Palmer amaranth populations during a time course, and (3) identify mesotrione metabolites including and beyond Phase I oxidation. The Palmer amaranth populations, SYNR1 and SYNR2, exhibited higher survival rates (100%), aboveground biomass (c.a. 50%), and lower injury (25-30%) following mesotrione treatment than other populations studied. These two populations also metabolized mesotrione 2-fold faster than sensitive populations, PPI1 and PPI2, and rapidly formed 4-OH-mesotrione. Additionally, SYNR1 and SYNR2 formed 5-OH-mesotrione, which is not produced in high abundance in waterhemp or naturally tolerant maize. Metabolite features derived from 4/5-OH-mesotrione and potential Phase II mesotrione-conjugates were detected and characterized by liquid chromatography-mass spectrometry (LCMS).
Keywords: cytochrome P450 monooxygenase; dioecious amaranth; herbicide detoxification; mesotrione resistance; metabolomics; oxidative metabolism; waterhemp.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Tembotrione detoxification in 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor-resistant Palmer amaranth (Amaranthus palmeri S. Wats.).Pest Manag Sci. 2018 Oct;74(10):2325-2334. doi: 10.1002/ps.4786. Epub 2018 Jan 5. Pest Manag Sci. 2018. PMID: 29105299
-
Distinct detoxification mechanisms confer resistance to mesotrione and atrazine in a population of waterhemp.Plant Physiol. 2013 Sep;163(1):363-77. doi: 10.1104/pp.113.223156. Epub 2013 Jul 19. Plant Physiol. 2013. PMID: 23872617 Free PMC article.
-
Overlapping Residual Herbicides for Control of Photosystem (PS) II- and 4-Hydroxyphenylpyruvate Dioxygenase (HPPD)-Inhibitor-Resistant Palmer amaranth (Amaranthus palmeri S. Watson) in Glyphosate-Resistant Maize.Front Plant Sci. 2018 Jan 9;8:2231. doi: 10.3389/fpls.2017.02231. eCollection 2017. Front Plant Sci. 2018. PMID: 29375605 Free PMC article.
-
Physiological and Molecular Characterization of Hydroxyphenylpyruvate Dioxygenase (HPPD)-inhibitor Resistance in Palmer Amaranth (Amaranthus palmeri S.Wats.).Front Plant Sci. 2017 Apr 11;8:555. doi: 10.3389/fpls.2017.00555. eCollection 2017. Front Plant Sci. 2017. PMID: 28443128 Free PMC article.
-
Physiological and Molecular Mechanisms of Differential Sensitivity of Palmer Amaranth (Amaranthus palmeri) to Mesotrione at Varying Growth Temperatures.PLoS One. 2015 May 19;10(5):e0126731. doi: 10.1371/journal.pone.0126731. eCollection 2015. PLoS One. 2015. PMID: 25992558 Free PMC article.
Cited by
-
Comparative metabolomics and transcriptomics provide new insights into florpyrauxifen-benzyl resistance in Echinochloa glabrescens.Front Plant Sci. 2024 Jul 3;15:1392460. doi: 10.3389/fpls.2024.1392460. eCollection 2024. Front Plant Sci. 2024. PMID: 39022606 Free PMC article.
References
-
- Ward S. M.; Webster T. M.; Steckel L. E. Palmer amaranth (Amaranthus palmeri): a review. Weed Technol. 2013, 27, 12–27. 10.1614/WT-D-12-00113.1. - DOI
-
- Keeley P. E.; Thullen R. J. Growth and competition of black nightshade (Solanum nigrum) and Palmer amaranth (Amaranthus palmeri) with cotton (Gossypium hirsutum). Weed Sci. 1989, 37, 326–334. 10.1017/S0043174500072003. - DOI
-
- Klingaman T. E.; Oliver L. R. Palmer amaranth (Amaranthus palmeri) interference in soybeans (Glycine max). Weed Sci. 1994, 42, 523–527. 10.1017/S0043174500076888. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

