Droplet microfluidics for time-resolved serial crystallography
- PMID: 38446456
- PMCID: PMC10916287
- DOI: 10.1107/S2052252524001799
Droplet microfluidics for time-resolved serial crystallography
Abstract
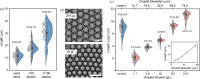
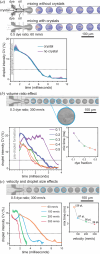
Serial crystallography requires large numbers of microcrystals and robust strategies to rapidly apply substrates to initiate reactions in time-resolved studies. Here, we report the use of droplet miniaturization for the controlled production of uniform crystals, providing an avenue for controlled substrate addition and synchronous reaction initiation. The approach was evaluated using two enzymatic systems, yielding 3 µm crystals of lysozyme and 2 µm crystals of Pdx1, an Arabidopsis enzyme involved in vitamin B6 biosynthesis. A seeding strategy was used to overcome the improbability of Pdx1 nucleation occurring with diminishing droplet volumes. Convection within droplets was exploited for rapid crystal mixing with ligands. Mixing times of <2 ms were achieved. Droplet microfluidics for crystal size engineering and rapid micromixing can be utilized to advance time-resolved serial crystallography.
Keywords: crystal miniaturization; droplet microfluidics; micromixing; time-resolved serial crystallography.
open access.
Figures

References
-
- Agirre, J., Atanasova, M., Bagdonas, H., Ballard, C. B., Baslé, A., Beilsten-Edmands, J., Borges, R. J., Brown, D. G., Burgos-Mármol, J. J., Berrisford, J. M., Bond, P. S., Caballero, I., Catapano, L., Chojnowski, G., Cook, A. G., Cowtan, K. D., Croll, T. I., Debreczeni, J. É., Devenish, N. E., Dodson, E. J., Drevon, T. R., Emsley, P., Evans, G., Evans, P. R., Fando, M., Foadi, J., Fuentes-Montero, L., Garman, E. F., Gerstel, M., Gildea, R. J., Hatti, K., Hekkelman, M. L., Heuser, P., Hoh, S. W., Hough, M. A., Jenkins, H. T., Jiménez, E., Joosten, R. P., Keegan, R. M., Keep, N., Krissinel, E. B., Kolenko, P., Kovalevskiy, O., Lamzin, V. S., Lawson, D. M., Lebedev, A. A., Leslie, A. G. W., Lohkamp, B., Long, F., Malý, M., McCoy, A. J., McNicholas, S. J., Medina, A., Millán, C., Murray, J. W., Murshudov, G. N., Nicholls, R. A., Noble, M. E. M., Oeffner, R., Pannu, N. S., Parkhurst, J. M., Pearce, N., Pereira, J., Perrakis, A., Powell, H. R., Read, R. J., Rigden, D. J., Rochira, W., Sammito, M., Sánchez Rodríguez, F., Sheldrick, G. M., Shelley, K. L., Simkovic, F., Simpkin, A. J., Skubak, P., Sobolev, E., Steiner, R. A., Stevenson, K., Tews, I., Thomas, J. M. H., Thorn, A., Valls, J. T., Uski, V., Usón, I., Vagin, A., Velankar, S., Vollmar, M., Walden, H., Waterman, D., Wilson, K. S., Winn, M. D., Winter, G., Wojdyr, M. & Yamashita, K. (2023). Acta Cryst. D79, 449–461.
-
- Akella, S. V., Mowitz, A., Heymann, M. & Fraden, S. (2014). Cryst. Growth Des. 14, 4487–4509.
-
- Bar-Even, A., Noor, E., Savir, Y., Liebermeister, W., Davidi, D., Tawfik, D. S. & Milo, R. (2011). Biochemistry, 50, 4402–4410. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

