Invasive Treatment Strategy in Adults With Frailty and Non-ST-Segment Elevation Myocardial Infarction: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 38446482
- PMCID: PMC10918507
- DOI: 10.1001/jamanetworkopen.2024.0809
Invasive Treatment Strategy in Adults With Frailty and Non-ST-Segment Elevation Myocardial Infarction: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: The MOSCA-FRAIL randomized clinical trial compared invasive and conservative treatment strategies in patients with frailty with non-ST-segment elevation myocardial infarction (NSTEMI). It showed no differences in the number of days alive and out of the hospital at 1 year.
Objective: To assess the outcomes of the MOSCA-FRAIL trial during extended follow-up.
Design, setting, and participants: The MOSCA-FRAIL randomized clinical trial was conducted at 13 hospitals in Spain between July 7, 2017, and January 9, 2021, and included 167 adults (aged ≥70 years) with frailty (Clinical Frailty Scale score ≥4) and NSTEMI. In this preplanned secondary analysis, follow-up was extended to January 31, 2023. Data analysis was performed from April 5 to 29, 2023, using the intention-to-treat principle.
Interventions: Patients were randomized to a routine invasive (coronary angiography and revascularization if feasible [n = 84]) or a conservative (medical treatment with coronary angiography only if recurrent ischemia [n = 83]) strategy.
Main outcomes and measures: The primary end point was the difference in restricted mean survival time (RMST). Secondary end points included readmissions for any cause, considering recurrent readmissions.
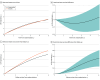
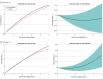
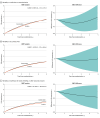
Results: Among the 167 patients included in the analysis, the mean (SD) age was 86 (5) years; 79 (47.3%) were men and 88 (52.7%) were women. A total of 93 deaths and 367 readmissions accrued. The RMST for all-cause death over the entire follow-up was 3.13 (95% CI, 2.72-3.60) years in the invasive and 3.06 (95% CI, 2.84-3.32) years in the conservative treatment groups. The RMST analysis showed inconclusive differences in survival time (invasive minus conservative difference, 28 [95% CI, -188 to 230] days). Patients under invasive treatment tended to have shorter survival in the first year (-28 [95% CI, -63 to 7] days), which improved after the first year (192 [95% CI, 90-230] days). Kaplan-Meier mortality curves intersected, displaying higher mortality to 1 year in the invasive group that shifted to a late benefit (landmark analysis hazard ratio, 0.58 [95% CI, 0.33-0.99]; P = .045). Early harm was more evident in the subgroup with a Clinical Frailty Scale score greater than 4. No differences were found for the secondary end points.
Conclusions and relevance: In this extended follow-up of a randomized clinical trial of patients with frailty and NSTEMI, an invasive treatment strategy did not improve outcomes at a median follow-up of 1113 (IQR, 443-1441) days. However, a differential distribution of deaths was observed, with early harm followed by later benefit. The phenomenon of depletion of susceptible patients may be responsible for this behavior.
Trial registration: ClinicalTrials.gov Identifier: NCT03208153.
Conflict of interest statement
Figures

References
-
- Damluji AA, Forman DE, Wang TY, et al. ; American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Lifestyle and Cardiometabolic Health . Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart Association. Circulation. 2023;147(3):e32-e62. doi:10.1161/CIR.0000000000001112 - DOI - PMC - PubMed
-
- Savonitto S, Cavallini C, Petronio AS, et al. ; Italian Elderly ACS Trial Investigators . Early aggressive versus initially conservative treatment in elderly patients with non–ST-segment elevation acute coronary syndrome: a randomized controlled trial. JACC Cardiovasc Interv. 2012;5(9):906-916. doi:10.1016/j.jcin.2012.06.008 - DOI - PubMed
-
- Tegn N, Abdelnoor M, Aaberge L, et al. ; After Eighty study investigators . Invasive versus conservative strategy in patients aged 80 years or older with non–ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open-label randomised controlled trial. Lancet. 2016;387(10023):1057-1065. doi:10.1016/S0140-6736(15)01166-6 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

