Ex vivo expansion potential of murine hematopoietic stem cells is a rare property only partially predicted by phenotype
- PMID: 38446538
- PMCID: PMC10942641
- DOI: 10.7554/eLife.91826
Ex vivo expansion potential of murine hematopoietic stem cells is a rare property only partially predicted by phenotype
Abstract
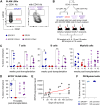
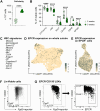
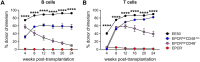
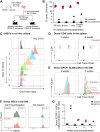
The scarcity of hematopoietic stem cells (HSCs) restricts their use in both clinical settings and experimental research. Here, we examined a recently developed method for expanding rigorously purified murine HSCs ex vivo. After 3 weeks of culture, only 0.1% of cells exhibited the input HSC phenotype, but these accounted for almost all functional long-term HSC activity. Input HSCs displayed varying potential for ex vivo self-renewal, with alternative outcomes revealed by single-cell multimodal RNA and ATAC sequencing profiling. While most HSC progeny offered only transient in vivo reconstitution, these cells efficiently rescued mice from lethal myeloablation. The amplification of functional HSC activity allowed for long-term multilineage engraftment in unconditioned hosts that associated with a return of HSCs to quiescence. Thereby, our findings identify several key considerations for ex vivo HSC expansion, with major implications also for assessment of normal HSC activity.
Keywords: ex vivo expansion; hematopoietic stem cell; mouse; regenerative medicine; stem cells; transplantation.
© 2023, Zhang et al.
Conflict of interest statement
QZ, RO, AK, OY, DB No competing interests declared
Figures













Update of
- doi: 10.1101/2022.06.20.496822
- doi: 10.7554/eLife.91826.1
- doi: 10.7554/eLife.91826.2
References
-
- Barnett D, Janossy G, Lubenko A, Matutes E, Newland A, Reilly JT. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells: prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clinical and Laboratory Haematology. 1999;21:301–308. doi: 10.1046/j.1365-2257.1999.00253.x. - DOI - PubMed
-
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, Treloar D, Brinkman RR, Eaves CJ. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

