Enduring Consensus Guidelines for Cervical Cancer Screening and Management: Introduction to the Scope and Process
- PMID: 38446573
- PMCID: PMC11520335
- DOI: 10.1097/LGT.0000000000000804
Enduring Consensus Guidelines for Cervical Cancer Screening and Management: Introduction to the Scope and Process
Abstract
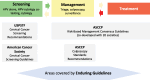
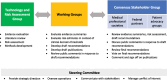
Objectives: The Enduring Consensus Cervical Cancer Screening and Management Guidelines (Enduring Guidelines) effort is a standing committee to continuously evaluate new technologies and approaches to cervical cancer screening, management, and surveillance.
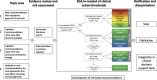
Methods and results: The Enduring Guidelines process will selectively incorporate new technologies and approaches with adequate supportive data to more effectively improve cancer prevention for high-risk individuals and decrease unnecessary procedures in low-risk individuals. This manuscript describes the structure, process, and methods of the Enduring Guidelines effort. Using systematic literature reviews and primary data sources, risk of precancer will be estimated and recommendations will be made based on risk estimates in the context of established risk-based clinical action thresholds. The Enduring Guidelines process will consider health equity and health disparities by assuring inclusion of diverse populations in the evidence review and risk assessment and by developing recommendations that provide a choice of well-validated strategies that can be adapted to different settings.
Conclusions: The Enduring Guidelines process will allow updating existing cervical cancer screening and management guidelines rapidly when new technologies are approved or new scientific evidence becomes available.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the ASCCP.
Conflict of interest statement
N.B. has served as a consultant for Dysis. M.E. has served as a consultant for Papivax, Merck, BD, and PDS. R.G. has served as a consultant for Inovio. D.H. has served as a consultant for Roche. W.H. has served as a consultant for Roche, SeeGene, and AstraZeneca. R.N. has participated in an educational meeting supported by Roche. The other authors have declared they have no conflicts of interest.
Figures
References
-
- Wentzensen N Arbyn M Berkhof J, et al. Eurogin 2016 roadmap: how HPV knowledge is changing screening practice. Int J Cancer 2017;140:2192–200. - PubMed
-
- Fontham ETH Wolf AMD Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin 2020;70:321–46. - PubMed
-
- US Preventive Services Task Force, Curry SJ Krist AH Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674–86. - PubMed

