Diagnostic accuracy of serum matrix metalloproteinase-7 as a biomarker of biliary atresia in a large North American cohort
- PMID: 38446707
- PMCID: PMC11191042
- DOI: 10.1097/HEP.0000000000000827
Diagnostic accuracy of serum matrix metalloproteinase-7 as a biomarker of biliary atresia in a large North American cohort
Abstract
Background and aims: High levels of serum matrix metalloproteinase-7 (MMP-7) have been linked to biliary atresia (BA), with wide variation in concentration cutoffs. We investigated the accuracy of serum MMP-7 as a diagnostic biomarker in a large North American cohort.
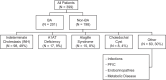
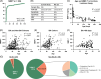
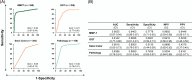
Approach and results: MMP-7 was measured in serum samples of 399 infants with cholestasis in the Prospective Database of Infants with Cholestasis study of the Childhood Liver Disease Research Network, 201 infants with BA and 198 with non-BA cholestasis (age median: 64 and 59 days, p = 0.94). MMP-7 was assayed on antibody-bead fluorescence (single-plex) and time resolved fluorescence energy transfer assays. The discriminative performance of MMP-7 was compared with other clinical markers. On the single-plex assay, MMP-7 generated an AUROC of 0.90 (CI: 0.87-0.94). At cutoff 52.8 ng/mL, it produced sensitivity = 94.03%, specificity = 77.78%, positive predictive value = 64.46%, and negative predictive value = 96.82% for BA. AUROC for gamma-glutamyl transferase = 0.81 (CI: 0.77-0.86), stool color = 0.68 (CI: 0.63-0.73), and pathology = 0.84 (CI: 0.76-0.91). Logistic regression models of MMP-7 with other clinical variables individually or combined showed an increase for MMP-7+gamma-glutamyl transferase AUROC to 0.91 (CI: 0.88-0.95). Serum concentrations produced by time resolved fluorescence energy transfer differed from single-plex, with an optimal cutoff of 18.2 ng/mL. Results were consistent within each assay technology and generated similar AUROCs.
Conclusions: Serum MMP-7 has high discriminative properties to differentiate BA from other forms of neonatal cholestasis. MMP-7 cutoff values vary according to assay technology. Using MMP-7 in the evaluation of infants with cholestasis may simplify diagnostic algorithms and shorten the time to hepatoportoenterostomy.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Sanjiv Harpavat has other interests with Syneos Health. Jean P. Molleston received grants from AbbVie, Albireo, CF Foundation, Gilead, and Mirum. Philip Rosenthal consults, is on the speakers’ bureau, and received grants from Mirum. He consults and received grants from Albireo, Dicerna, Takeda, and Traverse. He consults for Audentes, Encoded, and Taysha. He received grants from Arrowhead and Gilead. Ronald J. Sokol advises Albireo and Mirum. He is on the speakers’ bureau for Astellas. Evelyn K. Hsu received grants from Albireo, Gi, and Mirum. Saul J. Karpen consults for Albireo, HemoShear, Intercept, and Mirum. Kathleen M. Loomes consults and received grants from Albireo and Mirum. She consults for Travere. Simon P. Horslen consults for Albireo, Alexion, and iECURE. He received grants from Mirum. The remaining authors have no conflicts to report.
Figures


References
-
- Lakshminarayanan B, Davenport M. Biliary atresia: A comprehensive review. J Autoimmun. 2016;73:1–9. - PubMed
-
- Feldman AG, Mack CL. Biliary atresia: Clinical lessons learned. J Pediatr Gastroenterol Nutr. 2015;61:167–75. - PubMed
-
- Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, et al. . Biliary atresia: Current concepts and research directions. Summary of a symposium Hepatology. 1996;23:1682–92. - PubMed
-
- Perlmutter DH, Shepherd RW. Extrahepatic biliary atresia: A disease or a phenotype? Hepatology. 2002;35:1297–304. - PubMed
-
- Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: Current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK062481/DK/NIDDK NIH HHS/United States
- U01 DK062470/DK/NIDDK NIH HHS/United States
- U01 DK062436/DK/NIDDK NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- U01 DK062466/DK/NIDDK NIH HHS/United States
- U01 DK084575/DK/NIDDK NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01 DK084536/DK/NIDDK NIH HHS/United States
- U01 DK062456/DK/NIDDK NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- U01 DK103149/DK/NIDDK NIH HHS/United States
- UL1 TR000130/TR/NCATS NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- T32 DK007727/DK/NIDDK NIH HHS/United States
- U01 DK103140/DK/NIDDK NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- U01 DK084538/DK/NIDDK NIH HHS/United States
- U01 DK062453/DK/NIDDK NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- U01 DK103135/DK/NIDDK NIH HHS/United States
- U24 DK062456/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK062500/DK/NIDDK NIH HHS/United States
- U01 DK062497/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

