Impact of Nicotine Replacement Therapy Sampling on Cessation-Related Processes
- PMID: 38446860
- PMCID: PMC11290993
- DOI: 10.1097/ADM.0000000000001298
Impact of Nicotine Replacement Therapy Sampling on Cessation-Related Processes
Abstract
Objectives: Smoking prevalence remains high among low-income smokers. Understanding processes (eg, withdrawal, craving, motivation) in early smoking cessation is crucially important for designing effective interventions for this population.
Methods: This is a secondary analysis of a novel, in-session sampling intervention (ie, In Vivo) as compared with standard care behavioral smoking cessation counseling (SC) among community-dwelling low-income smokers (n = 83). This analysis examined the effect of 5 in-session sampling interventions on cessation-related processes and perceived advantages or disadvantages of nicotine replacement therapy (NRT) products over time using daily diaries.
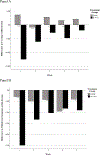
Results: The In Vivo treatment had an early positive impact in terms of decreasing withdrawal symptoms and cravings, and increasing perceived advantages to NRT, with moderate to large effect sizes. Results also showed that the treatment effectively reduced withdrawal symptoms and cravings in-session, with small-to-medium and medium-to-large effect sizes, respectively. In-session reduction of withdrawal symptoms and cravings did not occur for the SC group, with the exception of decreased withdrawal symptoms occurring during week 4. The In Vivo treatment did not impact quit goal, desire to quit, abstinence self-efficacy, perceived difficulty in quitting, motivational engagement, or perceived disadvantages to NRT. The In Vivo group reported less daily cigarette use relative to the SC group, in addition to reporting less cigarette use on days they reported greater combination NRT use.
Conclusions: There is preliminary support for this In Vivo treatment over SC in reducing withdrawal, craving, and the number of cigarettes smoked per day, as well as promoting perceived advantages of NRT among low-income smokers.
Trial registration: ClinicalTrials.gov NCT03276780.
Copyright © 2024 American Society of Addiction Medicine.
Conflict of interest statement
Figures
Similar articles
-
QuitNic: A Pilot Randomized Controlled Trial Comparing Nicotine Vaping Products With Nicotine Replacement Therapy for Smoking Cessation Following Residential Detoxification.Nicotine Tob Res. 2021 Feb 16;23(3):462-470. doi: 10.1093/ntr/ntaa143. Nicotine Tob Res. 2021. PMID: 32770246 Free PMC article. Clinical Trial.
-
Comparative effects of varenicline or combination nicotine replacement therapy versus patch monotherapy on candidate mediators of early abstinence in a smoking cessation attempt.Addiction. 2021 Apr;116(4):926-935. doi: 10.1111/add.15248. Epub 2020 Oct 1. Addiction. 2021. PMID: 32888230 Free PMC article. Clinical Trial.
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
-
Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2019 Apr 18;4(4):CD013308. doi: 10.1002/14651858.CD013308. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Jun 19;6:CD013308. doi: 10.1002/14651858.CD013308.pub2. PMID: 30997928 Free PMC article. Updated.
-
A Randomized Clinical Trial of Nicotine Preloading for Smoking Cessation in People with Posttraumatic Stress Disorder.J Dual Diagn. 2018 Jul-Sep;14(3):148-157. doi: 10.1080/15504263.2018.1468947. Epub 2018 Oct 10. J Dual Diagn. 2018. PMID: 29693495 Free PMC article. Clinical Trial.
References
-
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Centers for Disease Control and Prevention (US); 2014. Accessed August 22, 2022. http://www.ncbi.nlm.nih.gov/books/NBK179276/ - PubMed
-
- Santiago-Torres M, Mull KE, Sullivan BM, Kendzor DE, Bricker JB. Efficacy and utilization of smartphone applications for smoking cessation among low-income adults: Secondary analysis of the iCanQuit randomized trial. Drug Alcohol Depend 2022;231:109258. doi:10.1016/j.drugalcdep.2021.109258 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical