Machine learning predictions of T cell antigen specificity from intracellular calcium dynamics
- PMID: 38446885
- PMCID: PMC10917351
- DOI: 10.1126/sciadv.adk2298
Machine learning predictions of T cell antigen specificity from intracellular calcium dynamics
Abstract
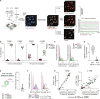
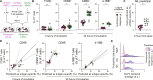
Adoptive T cell therapies rely on the production of T cells with an antigen receptor that directs their specificity toward tumor-specific antigens. Methods for identifying relevant T cell receptor (TCR) sequences, predominantly achieved through the enrichment of antigen-specific T cells, represent a major bottleneck in the production of TCR-engineered cell therapies. Fluctuation of intracellular calcium is a proximal readout of TCR signaling and candidate marker for antigen-specific T cell identification that does not require T cell expansion; however, calcium fluctuations downstream of TCR engagement are highly variable. We propose that machine learning algorithms may allow for T cell classification from complex datasets such as polyclonal T cell signaling events. Using deep learning tools, we demonstrate accurate prediction of TCR-transgenic CD8+ T cell activation based on calcium fluctuations and test the algorithm against T cells bearing a distinct TCR as well as polyclonal T cells. This provides the foundation for an antigen-specific TCR sequence identification pipeline for adoptive T cell therapies.
Figures


Similar articles
-
Multi-positive contrastive learning-based cross-attention model for T cell receptor-antigen binding prediction.Comput Methods Programs Biomed. 2025 Aug;268:108797. doi: 10.1016/j.cmpb.2025.108797. Epub 2025 May 10. Comput Methods Programs Biomed. 2025. PMID: 40378554
-
TCR clustering by contrastive learning on antigen specificity.Brief Bioinform. 2024 Jul 25;25(5):bbae375. doi: 10.1093/bib/bbae375. Brief Bioinform. 2024. PMID: 39129361 Free PMC article.
-
T-cell receptor dynamics in digestive system cancers: a multi-layer machine learning approach for tumor diagnosis and staging.Front Immunol. 2025 Apr 8;16:1556165. doi: 10.3389/fimmu.2025.1556165. eCollection 2025. Front Immunol. 2025. PMID: 40264789 Free PMC article.
-
TCR-NK Cells: A Novel Source for Adoptive Immunotherapy of Cancer.Turk J Haematol. 2023 Feb 28;40(1):1-10. doi: 10.4274/tjh.galenos.2022.2022.0534. Epub 2023 Jan 31. Turk J Haematol. 2023. PMID: 36719099 Free PMC article. Review.
-
Genetically Engineered T Cells and Recombinant Antibodies to Target Intracellular Neoantigens: Current Status and Future Directions.Int J Mol Sci. 2024 Dec 17;25(24):13504. doi: 10.3390/ijms252413504. Int J Mol Sci. 2024. PMID: 39769267 Free PMC article. Review.
Cited by
-
Engineering CAR-T Therapeutics for Enhanced Solid Tumor Targeting.Adv Mater. 2025 Jun;37(23):e2414882. doi: 10.1002/adma.202414882. Epub 2025 Jan 2. Adv Mater. 2025. PMID: 39745117 Free PMC article. Review.
-
Evaluating chemical effects on human neural cells through calcium imaging and deep learning.iScience. 2024 Nov 1;27(12):111298. doi: 10.1016/j.isci.2024.111298. eCollection 2024 Dec 20. iScience. 2024. PMID: 39634567 Free PMC article.
References
-
- Zhong S., Malecek K., Johnson L. A., Yu Z., Vega-Saenz de Miera E., Darvishian F., McGary K., Huang K., Boyer J., Corse E., Shao Y., Rosenberg S. A., Restifo N. P., Osman I., Krogsgaard M., T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 110, 6973–6978 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

