Coxsackievirus infection induces direct pancreatic β cell killing but poor antiviral CD8+ T cell responses
- PMID: 38446892
- PMCID: PMC10917340
- DOI: 10.1126/sciadv.adl1122
Coxsackievirus infection induces direct pancreatic β cell killing but poor antiviral CD8+ T cell responses
Abstract
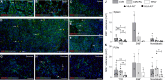
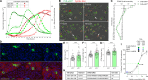
Coxsackievirus B (CVB) infection of pancreatic β cells is associated with β cell autoimmunity and type 1 diabetes. We investigated how CVB affects human β cells and anti-CVB T cell responses. β cells were efficiently infected by CVB in vitro, down-regulated human leukocyte antigen (HLA) class I, and presented few, selected HLA-bound viral peptides. Circulating CD8+ T cells from CVB-seropositive individuals recognized a fraction of these peptides; only another subfraction was targeted by effector/memory T cells that expressed exhaustion marker PD-1. T cells recognizing a CVB epitope cross-reacted with β cell antigen GAD. Infected β cells, which formed filopodia to propagate infection, were more efficiently killed by CVB than by CVB-reactive T cells. Our in vitro and ex vivo data highlight limited CD8+ T cell responses to CVB, supporting the rationale for CVB vaccination trials for type 1 diabetes prevention. CD8+ T cells recognizing structural and nonstructural CVB epitopes provide biomarkers to differentially follow response to infection and vaccination.
Figures





Update of
-
Coxsackievirus infection induces direct pancreatic β-cell killing but poor anti-viral CD8+ T-cell responses.bioRxiv [Preprint]. 2023 Aug 21:2023.08.19.553954. doi: 10.1101/2023.08.19.553954. bioRxiv. 2023. Update in: Sci Adv. 2024 Mar 8;10(10):eadl1122. doi: 10.1126/sciadv.adl1122. PMID: 37662376 Free PMC article. Updated. Preprint.
References
-
- Carré A., Vecchio F., Flodstrom-Tullberg M., You S., Mallone R., Coxsackievirus and type 1 diabetes: Diabetogenic mechanisms and implications for prevention. Endocr. Rev. 44, 737–751 (2023). - PubMed
-
- Gillespie K. M., Bain S. C., Barnett A. H., Bingley P. J., Christie M. R., Gill G. V., Gale E. A., The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 364, 1699–1700 (2004). - PubMed
-
- Patterson C. C., Harjutsalo V., Rosenbauer J., Neu A., Cinek O., Skrivarhaug T., Rami-Merhar B., Soltesz G., Svensson J., Parslow R. C., Castell C., Schoenle E. J., Bingley P. J., Dahlquist G., Jarosz-Chobot P. K., Marciulionyte D., Roche E. F., Rothe U., Bratina N., Ionescu-Tirgoviste C., Weets I., Kocova M., Cherubini V., Rojnic Putarek N., deBeaufort C. E., Samardzic M., Green A., Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: A multicentre prospective registration study. Diabetologia 62, 408–417 (2019). - PubMed
-
- Ziegler A.-G., Bonifacio E., BABYDIAB-BABYDIET Study Group , Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 55, 1937–1943 (2012). - PubMed
-
- Parikka V., Nanto-Salonen K., Saarinen M., Simell T., Ilonen J., Hyoty H., Veijola R., Knip M., Simell O., Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 55, 1926–1936 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

