Comparative Effectiveness of Natalizumab, Fingolimod, and Injectable Therapies in Pediatric-Onset Multiple Sclerosis: A Registry-Based Study
- PMID: 38447093
- PMCID: PMC11033984
- DOI: 10.1212/WNL.0000000000208114
Comparative Effectiveness of Natalizumab, Fingolimod, and Injectable Therapies in Pediatric-Onset Multiple Sclerosis: A Registry-Based Study
Erratum in
-
Corrections to Preprint Server Information.Neurology. 2024 Jul 9;103(1):e209573. doi: 10.1212/WNL.0000000000209573. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830142 Free PMC article. No abstract available.
Abstract
Background and objectives: Patients with pediatric-onset multiple sclerosis (POMS) typically experience higher levels of inflammation with more frequent relapses, and though patients with POMS usually recover from relapses better than adults, patients with POMS reach irreversible disability at a younger age than adult-onset patients. There have been few randomized, placebo-controlled clinical trials of multiple sclerosis (MS) disease-modifying therapies (DMTs) in patients with POMS, and most available data are based on observational studies of off-label use of DMTs approved for adults. We assessed the effectiveness of natalizumab compared with fingolimod using injectable platform therapies as a reference in pediatric patients in the global MSBase registry.
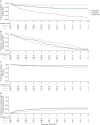
Methods: This retrospective study included patients with POMS who initiated treatment with an injectable DMT, natalizumab, or fingolimod between January 1, 2006, and May 3, 2021. Patients were matched using inverse probability treatment weighting. The primary outcome was time to first relapse from index therapy initiation. Secondary study outcomes included annualized relapse rate; proportions of relapse-free patients at 1, 2, and 5 years; time to treatment discontinuation; and times to 24-week confirmed disability worsening and confirmed disability improvement.
Results: A total of 1,218 patients with POMS were included in this analysis. Patients treated with fingolimod had a significantly lower risk of relapse than patients treated with injectable DMTs (hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.29-0.83; p = 0.008). After adjustment for prior DMT experience in the unmatched sample, patients treated with natalizumab had a significantly lower risk of relapse than patients treated either with injectable DMTs (HR, 0.15; 95% CI 0.07-0.31; p < 0.001) or fingolimod (HR, 0.37; 95% CI 0.14-1.00; p = 0.049). The adjusted secondary study outcomes were generally consistent with the primary outcome or with previous observations. The findings in the inverse probability treatment weighting-adjusted patient populations were confirmed in multiple sensitivity analyses.
Discussion: Our analyses of relapse risk suggest that natalizumab is more effective than fingolimod in the control of relapses in this population with high rates of new inflammatory activity, consistent with previous studies of natalizumab and fingolimod in adult-onset patients and POMS. In addition, both fingolimod and natalizumab were more effective than first-line injectable therapies.
Classification of evidence: This study provides Class II evidence that patients with POMS treated with natalizumab had a lower risk of relapse than those with fingolimod.
Conflict of interest statement
T. Spelman has received consulting fees and support for attending meetings and/or travel from Biogen and Novartis. G. Simoneau was employed by and may hold stock/stock options in Biogen. R. Hyde was employed by Biogen and may hold stock/stock options in Biogen. R. Kuhelj is an employee of and may hold stock/stock options in Biogen. R. Alroughani received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche and Sanofi-Genzyme. S. Ozakbas reports no disclosures relevant to the manuscript. R. Karabudak reports no disclosures relevant to the manuscript. B. Yamout reports no disclosures relevant to the manuscript. S. J. Khoury received compensation for scientific advisory board activity from Merck and Roche and is serving on IDMC for Biogen. M. Terzi received travel grants from Novartis, Bayer-Schering, Merck, and Teva; and has participated in clinical trials by Sanofi Aventis, Roche, and Novartis. C. Boz received conference travel support from Biogen, Novartis, Bayer-Schering, Merck, and Teva; and has participated in clinical trials by Sanofi Aventis, Roche, and Novartis. D. Horakova received compensation for travel, speaker honoraria, and consultant fees from Biogen, Novartis, Merck Healthcare KGaA (Darmstadt, Germany), Bayer, Sanofi, Roche, and Teva, as well as support for research activities from Biogen. She was also supported by the Charles University: Cooperation Program in Neuroscience. E.K. Havrdova received honoraria/research support from Biogen, Merck Serono, Novars, Roche, and Teva; and has been member of advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novars, and Sanofi Genzyme. B. Weinstock-Guttman has participated in speaker's bureaus and/or served as a consultant for Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, Bayer, Janssen, and Horizon. Dr. Weinstock-Guttman also has received grant/research support from the agencies listed in the previous sentence. She serves on the editorial board for BMJ Neurology, Children, CNS Drugs, MS International, and Frontiers Epidemiology. F. Patti received personal compensation for serving on advisory boards for Almirall, Alexion, Biogen, Bristol Myers Squibb, Merck, Novartis, and Roche. He has also received research grants from Biogen, Merck, Roche, FISM, Reload Association (Onlus), Italian Health Minister, and University of Catania. A. Altintas received speaker honoraria from Merck, Alexion; and received travel and registration grants from Merck. S. Mrabet has received a MENACTRIMS clinical fellowship grant (2020). J. Inshasi reports no disclosures relevant to the manuscript. V. Shaygannejad reports no disclosures relevant to the manuscript. S. Eichau has received speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck, Bayer, Sanofi Genzyme, Roche, and Teva. R. Gouider reports no disclosures relevant to the manuscript. W.L. Ward is an employee of Ashfield MedComms, an Inizio Company; medical writing support was funded by Biogen. H. Butzkueven has received grants or research support from Biogen, Roche, Merck, and Novartis; consulting fees from Oxford Health Policy Forum; and has received compensation or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Biogen, Roche, Merck, UCB, and Novartis. Go to
Figures
References
-
- Multiple Sclerosis International Federation. Atlas of MS 3rd edition. Part 2: clinical management of multiple sclerosis around the world; 2021. Accessed October 31, 2022. msif.org/wp-content/uploads/2021/05/Atlas-3rd-Edition-clinical-managemen....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
