ACE mRNA (Additional Chimeric Element incorporated IVT mRNA) for Enhancing Protein Expression by Modulating Immunogenicity
- PMID: 38447169
- PMCID: PMC11095206
- DOI: 10.1002/advs.202307541
ACE mRNA (Additional Chimeric Element incorporated IVT mRNA) for Enhancing Protein Expression by Modulating Immunogenicity
Abstract
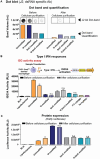
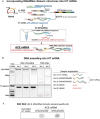
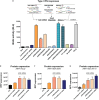
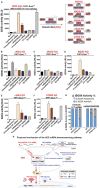
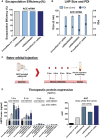
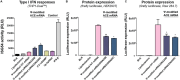
The development of in vitro transcribed mRNA (IVT mRNA)-based therapeutics/vaccines depends on the management of IVT mRNA immunogenicity. IVT mRNA, which is used for intracellular protein translation, often triggers unwanted immune responses, interfering with protein expression and leading to reduced therapeutic efficacy. Currently, the predominant approach for mitigating immune responses involves the incorporation of costly chemically modified nucleotides like pseudouridine (Ψ) or N1-methylpseudouridine (m1Ψ) into IVT mRNA, raising concerns about expense and the potential misincorporation of amino acids into chemically modified codon sequences. Here, an Additional Chimeric Element incorporated mRNA (ACE mRNA), a novel approach incorporating two segments within a single IVT mRNA structure, is introduced. The first segment retains conventional IVT mRNA components prepared with unmodified nucleotides, while the second, comprised of RNA/DNA chimeric elements, aims to modulate immunogenicity. Notably, ACE mRNA demonstrates a noteworthy reduction in immunogenicity of unmodified IVT mRNA, concurrently demonstrating enhanced protein expression efficiency. The reduced immune responses are based on the ability of RNA/DNA chimeric elements to restrict retinoic acid-inducible gene I (RIG-I) and stimulator of interferon genes (STING)-mediated immune activation. The developed ACE mRNA shows great potential in modulating the immunogenicity of IVT mRNA without the need for chemically modified nucleotides, thereby advancing the safety and efficacy of mRNA-based therapeutics/vaccines.
Keywords: RNA structure; immunogenicity; mRNA therapeutics; protein expression.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products.Cell Rep. 2022 Aug 30;40(9):111300. doi: 10.1016/j.celrep.2022.111300. Epub 2022 Aug 15. Cell Rep. 2022. PMID: 35988540 Free PMC article.
-
N1-methyl-pseudouridine is incorporated with higher fidelity than pseudouridine in synthetic RNAs.Sci Rep. 2022 Jul 29;12(1):13017. doi: 10.1038/s41598-022-17249-1. Sci Rep. 2022. PMID: 35906281 Free PMC article.
-
N1-methylpseudouridine-incorporated mRNA enhances exogenous protein expression and suppresses immunogenicity in primary human fibroblast-like synoviocytes.Cytotechnology. 2022 Aug;74(4):503-514. doi: 10.1007/s10616-022-00540-4. Epub 2022 Jun 30. Cytotechnology. 2022. PMID: 35791402 Free PMC article.
-
Immunogenicity of In Vitro-Transcribed RNA.Acc Chem Res. 2021 Nov 2;54(21):4012-4023. doi: 10.1021/acs.accounts.1c00521. Epub 2021 Oct 22. Acc Chem Res. 2021. PMID: 34677064 Free PMC article. Review.
-
Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems.Gene Ther. 2017 Mar;24(3):133-143. doi: 10.1038/gt.2017.5. Epub 2017 Jan 17. Gene Ther. 2017. PMID: 28094775 Review.
Cited by
-
Delivering the Message: Translating mRNA Therapy for Liver Inherited Metabolic Diseases.J Inherit Metab Dis. 2025 Sep;48(5):e70078. doi: 10.1002/jimd.70078. J Inherit Metab Dis. 2025. PMID: 40826824 Free PMC article. Review.
-
Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications.Signal Transduct Target Ther. 2024 Nov 14;9(1):322. doi: 10.1038/s41392-024-02002-z. Signal Transduct Target Ther. 2024. PMID: 39543114 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
