Fas2EB112: a tale of two chromosomes
- PMID: 38447284
- PMCID: PMC11075550
- DOI: 10.1093/g3journal/jkae047
Fas2EB112: a tale of two chromosomes
Abstract
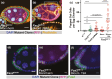
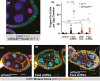
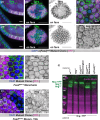
The cell-cell adhesion molecule Fasciclin II (Fas2) has long been studied for its evolutionarily conserved role in axon guidance. It is also expressed in the follicular epithelium, where together with a similar protein, Neuroglian (Nrg), it helps to drive the reintegration of cells born out of the tissue plane. Remarkably, one Fas2 protein null allele, Fas2G0336, demonstrates a mild reintegration phenotype, whereas work with the classic null allele Fas2EB112 showed more severe epithelial disorganization. These observations raise the question of which allele (if either) causes a bona fide loss of Fas2 protein function. The problem is not only relevant to reintegration but fundamentally important to understanding what this protein does and how it works: Fas2EB112 has been used in at least 37 research articles, and Fas2G0336 in at least three. An obvious solution is that one of the two chromosomes carries a modifier that either suppresses (Fas2G0336) or enhances (Fas2EB112) phenotypic severity. We find not only the latter to be the case, but identify the enhancing mutation as Nrg14, also a classic null allele.
Keywords: Drosophila; adhesion; epithelia; genetics.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The authors declare no conflict of interest.
Figures

Update of
-
Fas2EB112: A Tale of Two Chromosomes.bioRxiv [Preprint]. 2024 Jan 4:2024.01.03.574100. doi: 10.1101/2024.01.03.574100. bioRxiv. 2024. Update in: G3 (Bethesda). 2024 May 7;14(5):jkae047. doi: 10.1093/g3journal/jkae047. PMID: 38260405 Free PMC article. Updated. Preprint.
References
-
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. 1989. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 59(3):447–460. doi:10.1016/0092-8674(89)90029-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
