Excitability mediates allocation of pre-configured ensembles to a hippocampal engram supporting contextual conditioned threat in mice
- PMID: 38447576
- PMCID: PMC11065628
- DOI: 10.1016/j.neuron.2024.02.007
Excitability mediates allocation of pre-configured ensembles to a hippocampal engram supporting contextual conditioned threat in mice
Abstract
Little is understood about how engrams, sparse groups of neurons that store memories, are formed endogenously. Here, we combined calcium imaging, activity tagging, and optogenetics to examine the role of neuronal excitability and pre-existing functional connectivity on the allocation of mouse cornu ammonis area 1 (CA1) hippocampal neurons to an engram ensemble supporting a contextual threat memory. Engram neurons (high activity during recall or TRAP2-tagged during training) were more active than non-engram neurons 3 h (but not 24 h to 5 days) before training. Consistent with this, optogenetically inhibiting scFLARE2-tagged neurons active in homecage 3 h, but not 24 h, before conditioning disrupted memory retrieval, indicating that neurons with higher pre-training excitability were allocated to the engram. We also observed stable pre-configured functionally connected sub-ensembles of neurons whose activity cycled over days. Sub-ensembles that were more active before training were allocated to the engram, and their functional connectivity increased at training. Therefore, both neuronal excitability and pre-configured functional connectivity mediate allocation to an engram ensemble.
Keywords: activity tagging; allocation; calcium imaging; contextual fear conditioning; engram; excitability; hippocampus; memory; miniature endoscope.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.A.J. is a member of the advisory board of Neuron.
Figures
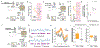
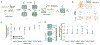

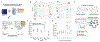
References
-
- Schacter DL (1982). Stranger behind the engram : theories of memory and the psychology (Erlbaum Associates; ).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

