CLU (clusterin) and PPARGC1A/PGC1α coordinately control mitophagy and mitochondrial biogenesis for oral cancer cell survival
- PMID: 38447939
- PMCID: PMC11210931
- DOI: 10.1080/15548627.2024.2309904
CLU (clusterin) and PPARGC1A/PGC1α coordinately control mitophagy and mitochondrial biogenesis for oral cancer cell survival
Abstract
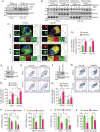
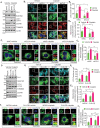
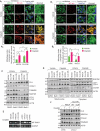
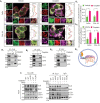
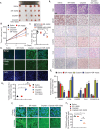
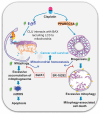
Mitophagy involves the selective elimination of defective mitochondria during chemotherapeutic stress to maintain mitochondrial homeostasis and sustain cancer growth. Here, we showed that CLU (clusterin) is localized to mitochondria to induce mitophagy controlling mitochondrial damage in oral cancer cells. Moreover, overexpression and knockdown of CLU establish its mitophagy-specific role, where CLU acts as an adaptor protein that coordinately interacts with BAX and LC3 recruiting autophagic machinery around damaged mitochondria in response to cisplatin treatment. Interestingly, CLU triggers class III phosphatidylinositol 3-kinase (PtdIns3K) activity around damaged mitochondria, and inhibition of mitophagic flux causes the accumulation of excessive mitophagosomes resulting in reactive oxygen species (ROS)-dependent apoptosis during cisplatin treatment in oral cancer cells. In parallel, we determined that PPARGC1A/PGC1α (PPARG coactivator 1 alpha) activates mitochondrial biogenesis during CLU-induced mitophagy to maintain the mitochondrial pool. Intriguingly, PPARGC1A inhibition through small interfering RNA (siPPARGC1A) and pharmacological inhibitor (SR-18292) treatment counteracts CLU-dependent cytoprotection leading to mitophagy-associated cell death. Furthermore, co-treatment of SR-18292 with cisplatin synergistically suppresses tumor growth in oral cancer xenograft models. In conclusion, CLU and PPARGC1A are essential for sustained cancer cell growth by activating mitophagy and mitochondrial biogenesis, respectively, and their inhibition could provide better therapeutic benefits against oral cancer.
Keywords: Clusterin; PPARGC1A/PGC1α; mitochondrial biogenesis; mitophagy; mitophagy-associated cell death.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
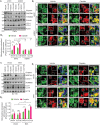

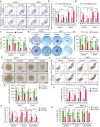

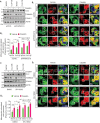
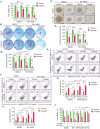
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
