Clinical outcomes and safety of immune checkpoint inhibitors in patients with solid tumors and paraneoplastic syndromes
- PMID: 38448038
- PMCID: PMC10916116
- DOI: 10.1136/jitc-2023-008724
Clinical outcomes and safety of immune checkpoint inhibitors in patients with solid tumors and paraneoplastic syndromes
Abstract
Background: Patients with paraneoplastic syndromes (PNS) are excluded from clinical trials involving immune checkpoint inhibitors (ICIs) due to safety concerns. Moreover, real-world data on efficacy and safety is scarce.
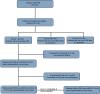
Methods: In this retrospective study, data were collected on patients with PNS and solid tumors receiving ICI between 2015 and 2022 at nine institutions. Patients were classified into: Cohort 1 (pre-existing PNS before ICI initiation), cohort 2 (PNS during ICI treatment), and cohort 3 (PNS after ICI discontinuation). Patients with metastatic non-small cell lung cancer (NSCLC) (mNSCLC) from cohort 1 were matched to patients who were PNS-free at each institution up to a 1:3 ratio for age, sex, type of ICI, use of concurrent chemotherapy, and number of lines of systemic therapy prior to ICI initiation. Kaplan-Meier method was used to assess overall survival (OS) and time-to-next treatment (TTNT).
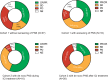
Results: Among 109 patients with PNS treated with ICIs, median age at ICI initiation was 67 years (IQR: 58-74). The most represented cancer type was NSCLC (n=39, 36%). In cohort 1 (n=55), PNS exacerbations occurred in 16 (29%) patients with median time to exacerbation after ICI of 1.1 months (IQR: 0.7-3.3). Exacerbation or de novo PNS prompted temporary/permanent interruption of ICIs in 14 (13%) patients. For cohort 2 (n=16), median time between ICI initiation and de novo PNS was 1.2 months (IQR: 0.4-3.5). Treatment-related adverse events (trAEs) occurred in 43 (39%) patients. Grade ≥3 trAEs occurred in 18 (17%) patients. PNS-directed immunosuppressive therapy was required in 55 (50%) patients. We matched 18 patients with mNSCLC and PNS (cohort 1) to 40 without PNS, treated with ICIs. There was no significant difference in OS or TTNT between patients with mNSCLC with and without PNS, although a trend was seen towards worse outcomes in patients with PNS. TrAEs occurred in 6/18 (33%) and 14/40 (35%), respectively. Grade ≥3 trAEs occurred in 4 (22%) patients with PNS and 7 (18%) patients without PNS.
Conclusions: Exacerbations of pre-existing PNS occurred in 29% of patients treated with ICIs and both exacerbations and de novo PNS occur early in the ICI course. TrAE from ICIs were similar between patients with and without PNS. Our data suggest that pre-existing PNS should not preclude consideration of ICI therapy although patients may not derive the same clinical benefit compared with patients without PNS.
Keywords: Immune Checkpoint Inhibitors; Non-Small Cell Lung Cancer; PARANEOPLASTIC SYNDROME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AHN receives honoraria from OncLive, TEMPUS, and Korean Society for Medical Oncology. Consulting fees: Guidepoint Global. RRM: Consulting/Advisory Board – Aveo, AstraZeneca, Bayer, Bristol Myers Squibb, Blue Earth Diagnostics, Calithera, Caris, Denderon, Exelixis, Janssen, Merck, Myovant, Pfizer, Sanofi, SeaGen, Sorrento Therapeutics, Tempus. Institutional Research Funding – AstraZeneca, BMS, Exelixis, Artera, Oncternal, Bayer, Tempus. JAS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers R01 AR080659, R01 AR077607, P30 AR070253, and P30 AR072577), the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award funded by the Gordon and Llura Gund Foundation. JAS has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, Pfizer, ReCor, and Sobi unrelated to this work. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health. AC received grants for consultancies/advisory boards: MSD, OncoC4, IQVIA, AstraZeneca, Access Infinity, Ardelis Health, Alpha Sight. Speaker fees: AstraZeneca, Eisai, Pierre-Fabre, MSD. Writing/Editorial activity: BMS. Travel support: Sanofi and MSD. ARN reports Funding to Institution for Trials he is PI on:Loxo@Lilly, Surface Oncology, ADC Therapeutics, IGM Biosciences, EMD Serono, Aravive, Nikang Therapeutics, Inspirna, Exelixis, Revolution Medicine, Jacobio, Pionyr, Jazz Pharmaceuticals, NGM Biopharmaceuticals. ARN receives Consultant Editor Compensation: JCO Precision Oncology. Consulting/Advisory Board: Foundation Med. ARN reports Travel Compensation from: SITC/ AACR/ Conquer Cancer Foundation, Jazz Pharmaceuticals, Binay Tara Foundation, Foundation Med.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
