Compatibility of Standard Vagus Nerve Stimulation and Investigational Microburst Vagus Nerve Stimulation Therapy with fMRI
- PMID: 38448165
- PMCID: PMC11383398
- DOI: 10.3174/ajnr.A8235
Compatibility of Standard Vagus Nerve Stimulation and Investigational Microburst Vagus Nerve Stimulation Therapy with fMRI
Abstract
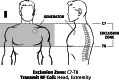
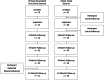
Vagus nerve stimulation devices are conditionally approved for MR imaging with stimulation turned off, and the requirement to modify the stimulation settings may be a barrier to scanning in some radiology practices. There is increasing interest in studying the effects of stimulation during MR imaging/fMRI. This study evaluated the safety of standard and investigational microburst vagus nerve stimulation therapies during MR imaging/fMRI. A prospective, multicenter study was conducted in patients with an investigational vagus nerve stimulation device that delivered either standard or investigational microburst vagus nerve stimulation. Thirty participants underwent sequential MR imaging and fMRI scans, encompassing 188 total hours of scan time (62.7 hours with standard vagus nerve stimulation and 125.3 hours with investigational microburst vagus nerve stimulation). No adverse events were reported with active stimulation during MR imaging or during 12 months of follow-up. Our results support the safety of standard and investigational microburst vagus nerve stimulation therapy during MR imaging and fMRI scans.
© 2024 by American Journal of Neuroradiology.
Figures

References
-
- Summary on safety and effectiveness data (SSED): VNS Therapy System. https://www.accessdata.fda.gov/cdrh_docs/pdf/p970003s207b.pdf. Accessed January 2, 2024
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
