Adaptive multi-interventional trial platform to improve patient care for fibrotic interstitial lung diseases
- PMID: 38448221
- PMCID: PMC11287572
- DOI: 10.1136/thorax-2023-221148
Adaptive multi-interventional trial platform to improve patient care for fibrotic interstitial lung diseases
Abstract
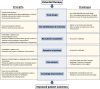
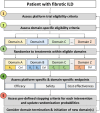
Background: Fibrotic interstitial lung diseases (fILDs) are a heterogeneous group of lung diseases associated with significant morbidity and mortality. Despite a large increase in the number of clinical trials in the last 10 years, current regulatory-approved management approaches are limited to two therapies that prevent the progression of fibrosis. The drug development pipeline is long and there is an urgent need to accelerate this process. This manuscript introduces the concept and design of an innovative research approach to drug development in fILD: a global Randomised Embedded Multifactorial Adaptive Platform in fILD (REMAP-ILD).
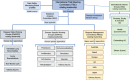
Methods: Description of the REMAP-ILD concept and design: the specific terminology, design characteristics (multifactorial, adaptive features, statistical approach), target population, interventions, outcomes, mission and values, and organisational structure.
Results: The target population will be adult patients with fILD, and the primary outcome will be a disease progression model incorporating forced vital capacity and mortality over 12 months. Responsive adaptive randomisation, prespecified thresholds for success and futility will be used to assess the effectiveness and safety of interventions. REMAP-ILD embraces the core values of diversity, equity, and inclusion for patients and researchers, and prioritises an open-science approach to data sharing and dissemination of results.
Conclusion: By using an innovative and efficient adaptive multi-interventional trial platform design, we aim to accelerate and improve care for patients with fILD. Through worldwide collaboration, novel analytical methodology and pragmatic trial delivery, REMAP-ILD aims to overcome major limitations associated with conventional randomised controlled trial approaches to rapidly improve the care of people living with fILD.
Keywords: Connective tissue disease associated lung disease; Hypersensitivity pneumonitis; Idiopathic pulmonary fibrosis; Interstitial Fibrosis; Rheumatoid lung disease.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LK-D—research grants from Boehringer Ingelheim and Bristol-Myers-Squibb, research grant from the Brazilian Ministry of Health (PROADI-SUS), non-financial research support from Fisher & Paykel; personal fees from Sarava and Boehringer Ingelheim. TK—personal fees from Boehringer Ingelheim, United Therapeutics, Puretech and Veracyte. CJR—research grant from Boehringer Ingelheim; personal fees from Boehringer Ingelheim, Pliant Therapeutics, AstraZeneca, Trevi Therapeutics, Hoffmann La Roche and Cipla. PR-O—research grants from Boehringer Ingelheim, Hoffmann La Roche, CSL Behring, FibroGen, Vicore Pharma, Gilead, Galecto and Chiesi; personal fees from Boehringer Ingelheim, Hoffmann La Roche, Cipla, Tecnofarma, Respiratory Effectiveness Group and The Limbic. NC—personal fees from Boehringer Ingelheim, Liminal Biosciences, Vicor Pharma, Bridge Biotherapeutics and Transcrip. MF-C—research grants from CSL Behring, Boehringer Ingelheim and Roche; personal fees from Boehringer Ingelheim, MSD, AstraZeneca and GSK. A-MH-V—research grants from Boehringer Ingelheim and Janssen; personal fees from ARXX, Boehringer Ingelheim, Janssen, Medscape, Roche, Genentech, Bayer, Lilly and Merck Sharp & Dohme. KAJ—research grants from University Hospital Foundation and Three Lakes Foundation; personal fees from Boehringer Ingelheim, Pliant, Thyron, Brainomix and Hoffman La Roche. YHK—research grants from NHMRC, MRFF, Air Liquide Healthcare, Austin Medical Research Foundation, Lung Foundation Australia/Thoracic Society of Australia and New Zealand, and RACP. SBM—research grants from Three Lakes Foundation, NIH/NHLBI, Merck, Boehringer Ingelheim, Pliant Therapeutics, American Thoracic Society, and National Scleroderma Foundation; personal fees from Wolters Kluwer, Roche, DevPro Biopharma, Gilead, Accendatech, Cowen and APIE Therapeutics. LP—research grants from Janssen and Ferrer; personal fees from Janssen, Ferrer, United Therapeutics, MSD and Liquidia. HP—research grants from Boehringer Ingelheim, AstraZeneca and Siemens; personal fees from Boehringer Ingelheim, Sanofi, Janssen, MSD, AstraZeneca and Chiesi. MM-M—research grants from Boehringer Ingelheim and Roche; personal fees from Boehringer Ingelheim, Ferrer and Chiesi. JST—research grant from Boehringer Ingelheim; personal fees from Boehringer Ingelheim and Aflofarm. SR—personal fees from Cipla and Boehringer Ingelheim. JJ—research grants from Gilead, Microsoft research and GSK; personal fees from Boehringer Ingelheim, Roche, GSK and Takeda. DR—research grants from NIHR; personal fees from OMass Therapeutics, Sosei Heptares and GSK. LGS—personal fees from Daiichi Sankyo and Chiesi. MK—research grants from Boehringer Ingelheim and Roche; personal fees from Nichtraucherhelden/Sanero, Boehringer Ingelheim and Roche. NK—research grants from Veracyte, Boehringer Ingelheim, BMS and non-financial support from AstraZeneca; scientific founder at Thyron; personal fees from Boehringer Ingelheim, Pliant, AstraZeneca, RohBar, Veracyte, Augmanity, CSL Behring, Splisense, Galapagos, Fibrogen, GSK, Merck and Thyron; and reports Equity in Pliant and Thyron. RL—senior consultant for Berry Consultants. WA—personal fees from Boehringer Ingelheim. GJ—research grants from AstraZeneca, Biogen, Galecto, GSK, Nordic Biosciences, RedX and Pliant; personal fees from Apollo Therapeutics, AstraZeneca, Brainomix, Bristol-Myers-Squibb, Chiesi, Cohbar, Daewoong, Veracyte, GSK, Resolution Therapeutics, Pliant, Hoffmann La Roche, PatientMPower, Pinsent Masons, Galapagos and Vicore Pharma. BGB, IB-V, BW, TJ, MQ and VC have no conflicts to declare.
Figures
References
-
- Travis WD, Costabel U, Hansell DM, et al. . An official American Thoracic society/European respiratory society statement: update of the International multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST - DOI - PMC - PubMed
-
- Dressen A, Abbas AR, Cabanski C, et al. . Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med 2018;6:603–14. 10.1016/S2213-2600(18)30135-8 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
