Capsules and their traits shape phage susceptibility and plasmid conjugation efficiency
- PMID: 38448399
- PMCID: PMC10918111
- DOI: 10.1038/s41467-024-46147-5
Capsules and their traits shape phage susceptibility and plasmid conjugation efficiency
Abstract
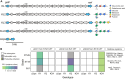
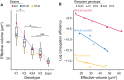
Bacterial evolution is affected by mobile genetic elements like phages and conjugative plasmids, offering new adaptive traits while incurring fitness costs. Their infection is affected by the bacterial capsule. Yet, its importance has been difficult to quantify because of the high diversity of confounding mechanisms in bacterial genomes such as anti-viral systems and surface receptor modifications. Swapping capsule loci between Klebsiella pneumoniae strains allowed us to quantify their impact on plasmid and phage infection independently of genetic background. Capsule swaps systematically invert phage susceptibility, revealing serotypes as key determinants of phage infection. Capsule types also influence conjugation efficiency in both donor and recipient cells, a mechanism shaped by capsule volume and conjugative pilus structure. Comparative genomics confirmed that more permissive serotypes in the lab correspond to the strains acquiring more conjugative plasmids in nature. The least capsule-sensitive pili (F-like) are the most frequent in the species' plasmids, and are the only ones associated with both antibiotic resistance and virulence factors, driving the convergence between virulence and antibiotics resistance in the population. These results show how traits of cellular envelopes define slow and fast lanes of infection by mobile genetic elements, with implications for population dynamics and horizontal gene transfer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kostina E, et al. Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D. Infect. Immun. 2005;73:8282–8290. doi: 10.1128/IAI.73.12.8282-8290.2005. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- ANR 18 CE12 0001 01 ENCAPSULATION/Agence Nationale de la Recherche (French National Research Agency)
- ANR 16 CE15 0022 03 PREDIRES/Agence Nationale de la Recherche (French National Research Agency)
- ANR-10-LABX-62- IBEID/Agence Nationale de la Recherche (French National Research Agency)
- PIA/ANR-16- CONV-0005/Agence Nationale de la Recherche (French National Research Agency)
- EQU201903007835/Fondation pour la Recherche Médicale (Foundation for Medical Research in France)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

