In situ electrochemical regeneration of nanogap hotspots for continuously reusable ultrathin SERS sensors
- PMID: 38448412
- PMCID: PMC10917746
- DOI: 10.1038/s41467-024-46097-y
In situ electrochemical regeneration of nanogap hotspots for continuously reusable ultrathin SERS sensors
Abstract
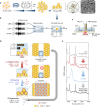
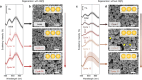
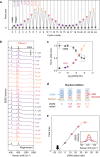
Surface-enhanced Raman spectroscopy (SERS) harnesses the confinement of light into metallic nanoscale hotspots to achieve highly sensitive label-free molecular detection that can be applied for a broad range of sensing applications. However, challenges related to irreversible analyte binding, substrate reproducibility, fouling, and degradation hinder its widespread adoption. Here we show how in-situ electrochemical regeneration can rapidly and precisely reform the nanogap hotspots to enable the continuous reuse of gold nanoparticle monolayers for SERS. Applying an oxidising potential of +1.5 V (vs Ag/AgCl) for 10 s strips a broad range of adsorbates from the nanogaps and forms a metastable oxide layer of few-monolayer thickness. Subsequent application of a reducing potential of -0.80 V for 5 s in the presence of a nanogap-stabilising molecular scaffold, cucurbit[5]uril, reproducibly regenerates the optimal plasmonic properties with SERS enhancement factors ≈106. The regeneration of the nanogap hotspots allows these SERS substrates to be reused over multiple cycles, demonstrating ≈5% relative standard deviation over at least 30 cycles of analyte detection and regeneration. Such continuous and reliable SERS-based flow analysis accesses diverse applications from environmental monitoring to medical diagnostics.
© 2024. The Author(s).
Conflict of interest statement
The authors J.J.B., S.M.S.-T., D.-B.G., M.N., and E.W. declare the following competing interests: filed patent, Surface-enhanced spectroscopy substrates, UK 2304765.7, 30/3/2023. The authors G.K., N.S., A.R., and B.d.N. declare no competing interests.
Figures


References
Grants and funding
- PICOFORCE (Grant Agreement No. 883703)/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- POSEIDON (Grant Agreement No. 861950)/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- EP/L015978/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/L027151/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- EP/X037770/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
LinkOut - more resources
Full Text Sources
Miscellaneous

