An artificial protein modulator reprogramming neuronal protein functions
- PMID: 38448420
- PMCID: PMC10917760
- DOI: 10.1038/s41467-024-46308-6
An artificial protein modulator reprogramming neuronal protein functions
Abstract
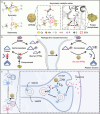
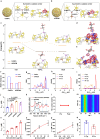
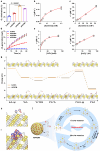
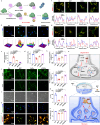
Reversible protein phosphorylation, regulated by protein phosphatases, fine-tunes target protein function and plays a vital role in biological processes. Dysregulation of this process leads to aberrant post-translational modifications (PTMs) and contributes to disease development. Despite the widespread use of artificial catalysts as enzyme mimetics, their direct modulation of proteins remains largely unexplored. To address this gap and enable the reversal of aberrant PTMs for disease therapy, we present the development of artificial protein modulators (APROMs). Through atomic-level engineering of heterogeneous catalysts with asymmetric catalytic centers, these modulators bear structural similarities to protein phosphatases and exhibit remarkable ability to destabilize the bridging μ3-hydroxide. This activation of catalytic centers enables spontaneous hydrolysis of phospho-substrates, providing precise control over PTMs. Notably, APROMs, with protein phosphatase-like characteristics, catalytically reprogram the biological function of α-synuclein by directly hydrolyzing hyperphosphorylated α-synuclein. Consequently, synaptic function is reinforced in Parkinson's disease. Our findings offer a promising avenue for reprogramming protein function through de novo PTMs strategy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson's disease: Potential contributions of environmental factors.Biochim Biophys Acta Mol Basis Dis. 2019 Aug 1;1865(8):1992-2000. doi: 10.1016/j.bbadis.2018.11.017. Epub 2018 Nov 24. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30481588 Free PMC article. Review.
-
Identification of novel protein phosphatases as modifiers of alpha-synuclein aggregation in yeast.FEMS Yeast Res. 2018 Dec 1;18(8). doi: 10.1093/femsyr/foy108. FEMS Yeast Res. 2018. PMID: 30277516
-
α-Synuclein phosphorylation as a therapeutic target in Parkinson's disease.Rev Neurosci. 2012 Mar 21;23(2):191-8. doi: 10.1515/revneuro-2011-0067. Rev Neurosci. 2012. PMID: 22499677 Review.
-
Inhibitors of Serine/Threonine Protein Phosphatases: Biochemical and Structural Studies Provide Insight for Further Development.Curr Med Chem. 2019;26(15):2634-2660. doi: 10.2174/0929867325666180508095242. Curr Med Chem. 2019. PMID: 29737249 Free PMC article. Review.
-
Targeting α-synuclein post-translational modifications in Parkinson's disease.Behav Brain Res. 2023 Feb 15;439:114204. doi: 10.1016/j.bbr.2022.114204. Epub 2022 Nov 11. Behav Brain Res. 2023. PMID: 36372243 Review.
Cited by
-
On the free energy of protein folding in optical tweezers experiments.Biophys Rev. 2025 Apr 22;17(2):231-245. doi: 10.1007/s12551-025-01310-0. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376413 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

