Synthesis and evaluation of coumarin derivatives on antioxidative, tyrosinase inhibitory activities, melanogenesis, and in silico investigations
- PMID: 38448547
- PMCID: PMC10917816
- DOI: 10.1038/s41598-024-54665-x
Synthesis and evaluation of coumarin derivatives on antioxidative, tyrosinase inhibitory activities, melanogenesis, and in silico investigations
Abstract
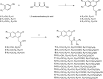
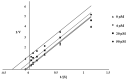
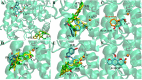
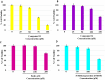
New coumarin derivatives were designed using a 2-(2-oxo-2H-chromen-4-yl)acetic acid scaffold conjugated with amino acid esters or tyramine. The anti-tyrosinase and anti-lipid peroxidation activities of the synthesized compounds were investigated. Coumarin derivatives 7,9, 11-13, 15-18 showed strong anti-lipid peroxidation activity. Compound 13 exhibited uncompetitive tyrosinase inhibitory activity with an IC50 value of 68.86 µM. Compound 14 (% activity = 123.41) showed stronger tyrosinase activating activity than 8-methoxypsolaren (8-MOP, % activity = 109.46). In silico studies revealed different poses between the inhibitors and activators near the tyrosinase catalytic site. Compounds 13 (25-50 μM) and 14 (25-100 μM) did not show cytotoxicity against B16F10 cells. In contrast to the tyrosinase inhibition assay, compound 13 (50 μM) suppressed melanogenesis in B16F10 cells with two times higher potency than KA (100 μM). Compound 14 at 100 μM showed melanogenesis enhancement in B16F10 cells in a dose-dependent manner, however, inferior to the 8-MOP. Based on the findings, compound 13 and 14 offer potential for development as skin-lightening agents and vitiligo therapy agents, respectively.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Urolithin and Reduced Urolithin Derivatives as Potent Inhibitors of Tyrosinase and Melanogenesis: Importance of the 4-Substituted Resorcinol Moiety.Int J Mol Sci. 2021 May 25;22(11):5616. doi: 10.3390/ijms22115616. Int J Mol Sci. 2021. PMID: 34070680 Free PMC article.
-
Design, synthesis of Cinnamyl-paeonol derivatives with 1, 3-Dioxypropyl as link arm and screening of tyrosinase inhibition activity in vitro.Bioorg Chem. 2021 Jan;106:104512. doi: 10.1016/j.bioorg.2020.104512. Epub 2020 Nov 24. Bioorg Chem. 2021. PMID: 33293056
-
Synthesis and discovery of potential tyrosinase inhibitor of new coumarin-based thiophenyl-pyrazolylthiazole nuclei: In vitro evaluation, cytotoxicity, kinetic, and computational studies.Chem Biol Drug Des. 2023 Jun;101(6):1262-1272. doi: 10.1111/cbdd.14209. Epub 2023 Feb 12. Chem Biol Drug Des. 2023. PMID: 36746678
-
Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo.Molecules. 2017 Aug 4;22(8):1303. doi: 10.3390/molecules22081303. Molecules. 2017. PMID: 28777326 Free PMC article. Review.
-
Critical review of Ayurvedic Varṇya herbs and their tyrosinase inhibition effect.Anc Sci Life. 2015 Jul-Sep;35(1):18-25. doi: 10.4103/0257-7941.165627. Anc Sci Life. 2015. PMID: 26600663 Free PMC article. Review.
Cited by
-
Bioprospection of indigenous herbal formulations for diabetes care: in vitro, network pharmacology, and molecular dynamics studies.BMC Complement Med Ther. 2025 Jul 3;25(1):225. doi: 10.1186/s12906-025-04980-1. BMC Complement Med Ther. 2025. PMID: 40611246 Free PMC article.
-
5,7-Dihydroxy-4-Methylcoumarin as a Functional Compound for Skin Pigmentation and Human Skin Safety.Pharmaceuticals (Basel). 2025 Mar 25;18(4):463. doi: 10.3390/ph18040463. Pharmaceuticals (Basel). 2025. PMID: 40283900 Free PMC article.
-
Design, Synthesis, Structural Insights, Tyrosinase Inhibition, and Sun Protection Factor of New Thiosemicarbazone Derivatives.Molecules. 2024 Nov 28;29(23):5629. doi: 10.3390/molecules29235629. Molecules. 2024. PMID: 39683787 Free PMC article.
-
Synergistic Biomedical Potential and Molecular Docking Analyses of Coumarin-Triazole Hybrids as Tyrosinase Inhibitors: Design, Synthesis, In Vitro Profiling, and In Silico Studies.Pharmaceuticals (Basel). 2024 Apr 20;17(4):532. doi: 10.3390/ph17040532. Pharmaceuticals (Basel). 2024. PMID: 38675492 Free PMC article.
-
Role of DNA Methylation in the Pathogenesis of Skin Disorders: Mechanisms, Inhibitors of Methylation-Related Enzyme, and Molecular Docking Studies.Biomed Res Int. 2025 Mar 27;2025:7002918. doi: 10.1155/bmri/7002918. eCollection 2025. Biomed Res Int. 2025. PMID: 40182929 Free PMC article. Review.
References
-
- Friedman M. Food browning and its prevention: An overview. J Agric. Food Chem. 1996;44:631–653. doi: 10.1021/jf950394r. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

