A vagal reflex evoked by airway closure
- PMID: 38448588
- PMCID: PMC10972749
- DOI: 10.1038/s41586-024-07144-2
A vagal reflex evoked by airway closure
Abstract
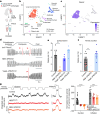
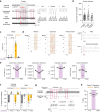
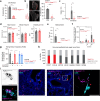
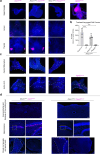
Airway integrity must be continuously maintained throughout life. Sensory neurons guard against airway obstruction and, on a moment-by-moment basis, enact vital reflexes to maintain respiratory function1,2. Decreased lung capacity is common and life-threatening across many respiratory diseases, and lung collapse can be acutely evoked by chest wall trauma, pneumothorax or airway compression. Here we characterize a neuronal reflex of the vagus nerve evoked by airway closure that leads to gasping. In vivo vagal ganglion imaging revealed dedicated sensory neurons that detect airway compression but not airway stretch. Vagal neurons expressing PVALB mediate airway closure responses and innervate clusters of lung epithelial cells called neuroepithelial bodies (NEBs). Stimulating NEBs or vagal PVALB neurons evoked gasping in the absence of airway threats, whereas ablating NEBs or vagal PVALB neurons eliminated gasping in response to airway closure. Single-cell RNA sequencing revealed that NEBs uniformly express the mechanoreceptor PIEZO2, and targeted knockout of Piezo2 in NEBs eliminated responses to airway closure. NEBs were dispensable for the Hering-Breuer inspiratory reflex, which indicated that discrete terminal structures detect airway closure and inflation. Similar to the involvement of Merkel cells in touch sensation3,4, NEBs are PIEZO2-expressing epithelial cells and, moreover, are crucial for an aspect of lung mechanosensation. These findings expand our understanding of neuronal diversity in the airways and reveal a dedicated vagal pathway that detects airway closure to help preserve respiratory function.
© 2024. The Author(s).
Conflict of interest statement
S.D.L. is a consultant for Kallyope. The remaining authors declare no competing interests.
Figures










References
-
- Coleridge, H. M. & Coleridge, J. C. G. in Comprehensive Physiology (ed. Terjung, R.) 395–429 (Wiley, 2011).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

