The structure and physical properties of a packaged bacteriophage particle
- PMID: 38448589
- PMCID: PMC11196859
- DOI: 10.1038/s41586-024-07150-4
The structure and physical properties of a packaged bacteriophage particle
Abstract
A string of nucleotides confined within a protein capsid contains all the instructions necessary to make a functional virus particle, a virion. Although the structure of the protein capsid is known for many virus species1,2, the three-dimensional organization of viral genomes has mostly eluded experimental probes3,4. Here we report all-atom structural models of an HK97 virion5, including its entire 39,732 base pair genome, obtained through multiresolution simulations. Mimicking the action of a packaging motor6, the genome was gradually loaded into the capsid. The structure of the packaged capsid was then refined through simulations of increasing resolution, which produced a 26 million atom model of the complete virion, including water and ions confined within the capsid. DNA packaging occurs through a loop extrusion mechanism7 that produces globally different configurations of the packaged genome and gives each viral particle individual traits. Multiple microsecond-long all-atom simulations characterized the effect of the packaged genome on capsid structure, internal pressure, electrostatics and diffusion of water, ions and DNA, and revealed the structural imprints of the capsid onto the genome. Our approach can be generalized to obtain complete all-atom structural models of other virus species, thereby potentially revealing new drug targets at the genome-capsid interface.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests
Figures




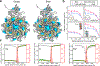




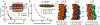





References
-
- Luque D, Castón JR. Cryo-electron microscopy for the study of virus assembly. Nat. Chem. Biol 16, 231–239 (2020). - PubMed
-
- Ilca S, Sun X, El Omari K, Kotecha A, de Haas F, DiMaio F, Grimes JM, Stuart DI, Poranen MM, Huiskonen JT. Multiple liquid crystalline geometries of highly compacted nucleic acid in a dsRNA virus. Nature 570, 252–256 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

