Cell-cycle inhibition and immune microenvironment in breast cancer treated with ribociclib and letrozole or chemotherapy
- PMID: 38448600
- PMCID: PMC10918094
- DOI: 10.1038/s41523-024-00625-7
Cell-cycle inhibition and immune microenvironment in breast cancer treated with ribociclib and letrozole or chemotherapy
Abstract
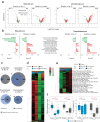
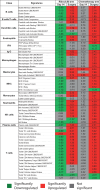
In this study, we performed genomic analyses of cell cycle and tumor microenvironment changes during and after ribociclib and letrozole or chemotherapy in the CORALLEEN trial. 106 women with untreated PAM50-defined Luminal B early breast cancers were randomly assigned to receive neoadjuvant ribociclib and letrozole or standard-of-care chemotherapy. Ki67 immunohistochemistry, tumor-infiltrating lymphocytes quantification, and RNA sequencing were obtained from tissue biopsies pre-treatment, on day 14 of treatment, and tumor specimens from surgical resection. Results showed that at surgery, Ki67 and the PAM50 proliferation scores were lower after ribociclib compared to chemotherapy. However, consistent reactivation of tumor cell proliferation from day 14 to surgery was only observed in the ribociclib arm. In tumors with complete cell cycle arrest (CCCA) at surgery, PAM50 proliferation scores were lower in the ribociclib arm compared to chemotherapy (p < 0.001), whereas the opposite was observed with tumor cellularity (p = 0.002). Gene expression signatures (GES) associated with antigen-presenting cells (APCs) and innate immune system activity showed increased expression post-chemotherapy but decreased expression post-ribociclib. Interferon-associated GES had decreased expression with CCCA and increased expression with non-CCCA. Our findings suggest that while both treatment strategies decreased proliferation, the depth and the patterns over time differed by treatment arm. Immunologically, ribociclib was associated with downregulated GES associated with APCs and the innate immune system in Luminal B tumors, contrary to existing preclinical data. Further studies are needed to understand the effect of CDK4/6 inhibition on the tumor cells and microenvironment, an effect which may vary according to tumor subtypes.
© 2024. The Author(s).
Conflict of interest statement
T.P. reports having received Fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers’ bureaus) from Astra Zeneca, Consulting Fees (e.g., advisory boards) from Novartis, and Fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers’ bureaus) from Pfizer. L.P. is an employee of Reveal Genomics and has a patent (EP 20 382 679.7—in vitro method for the prognosis of patients suffering from HER2-positive breast cancer). G.V. has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from MSD, GSK, Pierre Fabre, and Pfizer and has participated on a data safety monitoring board or advisory board for AstraZeneca. C.S. has served as consultant, participated in advisory boards or received travel grants from AstraZeneca, AX’s Consulting, Byondis BV, Celgene, Daiichi Sankyo, Eisai, F. Hoffmann - La Roche Ltd, Exact Sciences, Exeter Pharma, Genomic Health, Merck, Sharp and Dhome España S.A., Novartis, Odonate Therapeutics, Pfizer, Philips Healthwork, Pierre Fabre, Pint Pharma, prIME Oncology, Puma Biotechnology, Seagen, Synthon, Sanofi Aventis and Zymeworks. M.M. declares the following competing interests: expert testimony honoraria from Novartis, Roche, and Eisai; advisory board honoraria from Pierre Fabre, and Seagen; and conference travel grants from Roche, Pfizer, Gilead and Lilly. X.G. reports honoraria and personal fees from Roche, Novartis and Gilead, Roche, honoraria, travel support, personal fees from AstraZeneca and Pierre Fabre, and personal, travel and congress support from Eli Lilly. M.O. reports financial relationships with AstraZeneca, Guardant Health, Roche, MSD, Pfizer, SeaGen, iTEOS, Eisai, Novartis, Relay Therapeutics and Gilead. E.C. reports personal fees from Roche, personal fees from Lilly, personal fees from Novartis, personal fees from Pfizer, during the conduct of the study. J.G. reports grants and personal fees from NOVARTIS, grants and personal fees from PFIZER, grants and personal fees from ROCHE, outside the submitted work. A.P. has declared personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations, and expenses paid by Daiichi Sankyo, research funding from Nanostring Technologies, Roche and Novartis, consulting/advisory role for Nanostring Technologies, Roche, Novartis, Pfizer, Oncolytics Biotech, Amgen, Lilly, MSD, PUMA and Daiichi Sankyo, Inc. outside the submitted work. C.M.P. is an equity stockholder and consultant of BioClassifier LLC; C.M.P. is also listed as an inventor on patent applications for the Breast PAM50 Subtyping assay. The other authors declare no financial or non-financial competing interests.
Figures