Tektin bundle interacting protein, TEKTIP1, functions to stabilize the tektin bundle and axoneme in mouse sperm flagella
- PMID: 38448737
- PMCID: PMC10917850
- DOI: 10.1007/s00018-023-05081-3
Tektin bundle interacting protein, TEKTIP1, functions to stabilize the tektin bundle and axoneme in mouse sperm flagella
Abstract
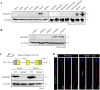
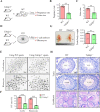
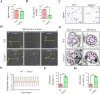
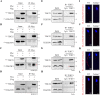
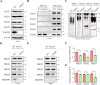
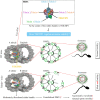
Tektins are microtubule inner proteins (MIPs) and localize at the inside lumen of doublet microtubules (DMTs) of cilia/flagella. TEKTIP1, a newly identified protein by cryo-electron microscopy (cryo-EM), is proposed to be localized at the center of the tektin bundle and hypothesized to recruit tektins or stabilize the bundle. However, the physiological role of TEKTIP1 is unknown. In this study, we generated Tektip1-knockout (Tektip1-/-) mice and showed that they were male subfertile primarily due to reduced sperm motility. A high percentage of sperm from Tektip1-/- mice showed moderately disorganized axoneme structures and abnormal flagellar waveforms. TEKTIP1 predominately interacted with TEKT3 among tektins. Loss of TEKTIP1 partially disturbed the organization of tektin bundle by mainly affecting the native status of TEKT3 and its interaction with other tektins. Collectively, our study reveals the physiological role and potential molecular mechanism of TEKTIP1 in axonemal structure and sperm motility, highlights the importance of MIPs in stabilizing DMTs, and suggests a potential relevance of TEKTIP1 deficiency to human asthenospermia. Tektip1-/- mice will be an excellent animal model to study the DMT organization of sperm flagella using cryo-EM in future.
Keywords: Knockout mice; Microtubule inner protein; Sperm motility; TEKTIP1; Tektins.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Brief biology and pathophysiology of Tekt bundles.Cell Adh Migr. 2025 Dec;19(1):2465421. doi: 10.1080/19336918.2025.2465421. Epub 2025 Feb 13. Cell Adh Migr. 2025. PMID: 39949046 Free PMC article. Review.
-
Tektin 3 is required for progressive sperm motility in mice.Mol Reprod Dev. 2009 May;76(5):453-9. doi: 10.1002/mrd.20957. Mol Reprod Dev. 2009. PMID: 18951373 Free PMC article.
-
Structures of sperm flagellar doublet microtubules expand the genetic spectrum of male infertility.Cell. 2023 Jun 22;186(13):2897-2910.e19. doi: 10.1016/j.cell.2023.05.009. Epub 2023 Jun 8. Cell. 2023. PMID: 37295417
-
Structural specializations of the sperm tail.Cell. 2023 Jun 22;186(13):2880-2896.e17. doi: 10.1016/j.cell.2023.05.026. Epub 2023 Jun 15. Cell. 2023. PMID: 37327785 Free PMC article.
-
Sperm flagella: comparative and phylogenetic perspectives of protein components.Mol Hum Reprod. 2011 Aug;17(8):524-38. doi: 10.1093/molehr/gar034. Epub 2011 May 17. Mol Hum Reprod. 2011. PMID: 21586547 Review.
Cited by
-
Motile Cilia in Female and Male Reproductive Tracts and Fertility.Cells. 2024 Nov 28;13(23):1974. doi: 10.3390/cells13231974. Cells. 2024. PMID: 39682722 Free PMC article. Review.
-
Brief biology and pathophysiology of Tekt bundles.Cell Adh Migr. 2025 Dec;19(1):2465421. doi: 10.1080/19336918.2025.2465421. Epub 2025 Feb 13. Cell Adh Migr. 2025. PMID: 39949046 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

