Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury
- PMID: 38448976
- PMCID: PMC10916222
- DOI: 10.1186/s12964-024-01539-4
Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury
Abstract
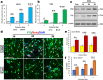
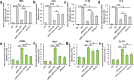
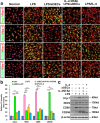
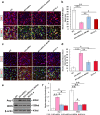
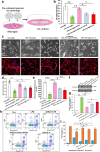
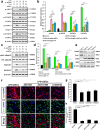
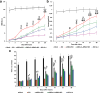
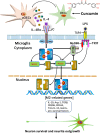
Microglia/macrophages are major contributors to neuroinflammation in the central nervous system (CNS) injury and exhibit either pro- or anti-inflammatory phenotypes in response to specific microenvironmental signals. Our latest in vivo and in vitro studies demonstrated that curcumin-treated olfactory ensheathing cells (aOECs) can effectively enhance neural survival and axonal outgrowth, and transplantation of aOECs improves the neurological outcome after spinal cord injury (SCI). The therapeutic effect is largely attributed to aOEC anti-inflammatory activity through the modulation of microglial polarization from the M1 to M2 phenotype. However, very little is known about what viable molecules from aOECs are actively responsible for the switch of M1 to M2 microglial phenotypes and the underlying mechanisms of microglial polarization. Herein, we show that Interleukin-4 (IL-4) plays a leading role in triggering the M1 to M2 microglial phenotype, appreciably decreasing the levels of M1 markers IL‑1β, IL‑6, tumour necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS) and elevating the levels of M2 markers Arg-1, TGF-β, IL-10, and CD206. Strikingly, blockade of IL-4 signaling by siRNA and a neutralizing antibody in aOEC medium reverses the transition of M1 to M2, and the activated microglia stimulated with the aOEC medium lacking IL-4 significantly decreases neuronal survival and neurite outgrowth. In addition, transplantation of aOECs improved the neurological function deficits after SCI in rats. More importantly, the crosstalk between JAK1/STAT1/3/6-targeted downstream signals and NF-κB/SOCS1/3 signaling predominantly orchestrates IL-4-modulated microglial polarization event. These results provide new insights into the molecular mechanisms of aOECs driving the M1-to-M2 shift of microglia and shed light on new therapies for SCI through the modulation of microglial polarization.
Keywords: Activated olfactory ensheathing cells; Interleukin-4; JAK1/STAT1/STAT3/STAT6; Microglia/macrophage polarization; Neuroinflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

