Antigenic drift and immunity gap explain reduction in protective responses against influenza A(H1N1)pdm09 and A(H3N2) viruses during the COVID-19 pandemic: a cross-sectional study of human sera collected in 2019, 2021, 2022, and 2023
- PMID: 38448981
- PMCID: PMC10916265
- DOI: 10.1186/s12985-024-02326-w
Antigenic drift and immunity gap explain reduction in protective responses against influenza A(H1N1)pdm09 and A(H3N2) viruses during the COVID-19 pandemic: a cross-sectional study of human sera collected in 2019, 2021, 2022, and 2023
Erratum in
-
Correction: antigenic drift and immunity gap explain reduction in protective responses against influenza A(H1N1)pdm09 and A(H3N2) viruses during the COVID-19 pandemic: a cross-sectional study of human sera collected in 2019, 2021, 2022, and 2023.Virol J. 2024 Mar 18;21(1):66. doi: 10.1186/s12985-024-02341-x. Virol J. 2024. PMID: 38500208 Free PMC article. No abstract available.
Abstract
Background: Non-pharmaceutical interventions implemented during the COVID-19 pandemic resulted in a marked reduction in influenza infections globally. The absence of influenza has raised concerns of waning immunity, and potentially more severe influenza seasons after the pandemic.
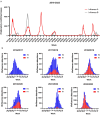
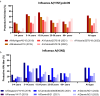
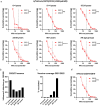
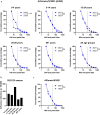
Methods: To evaluate immunity towards influenza post-COVID-19 pandemic we have assessed influenza A epidemics in Norway from October 2016 to June 2023 and measured antibodies against circulating strains of influenza A(H1N1)pdm09 and A(H3N2) in different age groups by hemagglutination inhibition (HAI) assays in a total of 3364 serum samples collected in 2019, 2021, 2022 and 2023.
Results: Influenza epidemics in Norway from October 2016 until June 2023 were predominately influenza As, with a mixture of A(H1N1)pdm09 and A(H3N2) subtype predominance. We did not observe higher numbers of infections during the influenza epidemics following the COVID-19 pandemic than in pre-COVID-19 seasons. Frequencies of protective HAI titers against A(H1N1)pdm09 and A(H3N2) viruses were reduced in sera collected in 2021 and 2022, compared to sera collected in 2019. The reduction could, however, largely be explained by antigenic drift of new virus strains, as protective HAI titers remained stable against the same strain from one season to the next. However, we observed the development of an immunity gap in the youngest children during the pandemic which resulted in a prominent reduction in HAI titers against A(H1N1)pdm09 in 2021 and 2022. The immunity gap was partially closed in sera collected in 2023 following the A(H1N1)pdm09-dominated influenza seasons of 2022/2023. During the 2022/2023 epidemic, drift variants of A(H1N1)pdm09 belonging to the 5a.2a.1 clade emerged, and pre-season HAI titers were significantly lower against this clade compared to the ancestral 5a.2 clade.
Conclusion: The observed reduction in protective antibodies against A(H1N1)pdm09 and A(H3N2) viruses post COVID-19 is best explained by antigenic drift of emerging viruses, and not waning of antibody responses in the general population. However, the absence of influenza during the pandemic resulted in an immunity gap in the youngest children. While this immunity gap was partially closed following the 2022/2023 influenza season, children with elevated risk of severe infection should be prioritized for vaccination.
Keywords: A(H1N1)pdm09; A(H3N2); Antibody; Antigenic drift; Immunity gap; Influenza; Serology.
© 2024. The Author(s).
Conflict of interest statement
The authors report no financial or other conflict of interest.
Figures
References
-
- Emborg HD, Carnahan A, Bragstad K, Trebbien R, Brytting M, Hungnes O, et al. Abrupt termination of the 2019/20 influenza season following preventive measures against COVID-19 in Denmark, Norway and Sweden. Euro Surveill. 2021;26(22):2001160. doi: 10.2807/1560-7917.ES.2021.26.22.2001160. - DOI - PMC - PubMed
-
- Folkehelseinstituttet, Risiko ved covid-19-epidemien, influensa og RSV-infeksjon i Norge. (2022)
-
- Weinberger Opek M, Yeshayahu Y, Glatman-Freedman A, Kaufman Z, Sorek N, Brosh-Nissimov T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Euro Surveill. 2021;26(29):2100706. doi: 10.2807/1560-7917.ES.2021.26.29.2100706. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

