Defects of mitochondria-lysosomes communication induce secretion of mitochondria-derived vesicles and drive chemoresistance in ovarian cancer cells
- PMID: 38448982
- PMCID: PMC10916030
- DOI: 10.1186/s12964-024-01507-y
Defects of mitochondria-lysosomes communication induce secretion of mitochondria-derived vesicles and drive chemoresistance in ovarian cancer cells
Abstract
Background: Among the mechanisms of mitochondrial quality control (MQC), generation of mitochondria-derived vesicles (MDVs) is a process to avoid complete failure of mitochondria determining lysosomal degradation of mitochondrial damaged proteins. In this context, RAB7, a late endocytic small GTPase, controls delivery of MDVs to late endosomes for subsequent lysosomal degradation. We previously demonstrated that RAB7 has a pivotal role in response to cisplatin (CDDP) regulating resistance to the drug by extracellular vesicle (EVs) secretion.
Methods: Western blot and immunofluorescence analysis were used to analyze structure and function of endosomes and lysosomes in CDDP chemosensitive and chemoresistant ovarian cancer cell lines. EVs were purified from chemosensitive and chemoresistant cells by ultracentrifugation or immunoisolation to analyze their mitochondrial DNA and protein content. Treatment with cyanide m-chlorophenylhydrazone (CCCP) and RAB7 modulation were used, respectively, to understand the role of mitochondrial and late endosomal/lysosomal alterations on MDV secretion. Using conditioned media from chemoresistant cells the effect of MDVs on the viability after CDDP treatment was determined. Seahorse assays and immunofluorescence analysis were used to study the biochemical role of MDVs and the uptake and intracellular localization of MDVs, respectively.
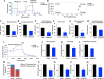
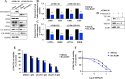
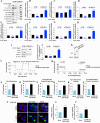
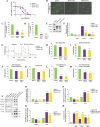
Results: We observed that CDDP-chemoresistant cells are characterized by increased MDV secretion, impairment of late endocytic traffic, RAB7 downregulation, an increase of RAB7 in EVs, compared to chemosensitive cells, and downregulation of the TFEB-mTOR pathway overseeing lysosomal and mitochondrial biogenesis and turnover. We established that MDVs can be secreted rather than delivered to lysosomes and are able to deliver CDDP outside the cells. We showed increased secretion of MDVs by chemoresistant cells ultimately caused by the extrusion of RAB7 in EVs, resulting in a dramatic drop in its intracellular content, as a novel mechanism to regulate RAB7 levels. We demonstrated that MDVs purified from chemoresistant cells induce chemoresistance in RAB7-modulated process, and, after uptake from recipient cells, MDVs localize to mitochondria and slow down mitochondrial activity.
Conclusions: Dysfunctional MQC in chemoresistant cells determines a block in lysosomal degradation of MDVs and their consequent secretion, suggesting that MQC is not able to eliminate damaged mitochondria whose components are secreted becoming effectors and potential markers of chemoresistance.
Keywords: Cisplatin chemoresistance; Extracellular vesicles; Mitochondria-derived vesicles; Ovarian cancer; RAB7.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

