Exploring the modulatory role of bovine lactoferrin on the microbiome and the immune response in healthy and Shiga toxin-producing E. coli challenged weaned piglets
- PMID: 38449023
- PMCID: PMC10916201
- DOI: 10.1186/s40104-023-00985-3
Exploring the modulatory role of bovine lactoferrin on the microbiome and the immune response in healthy and Shiga toxin-producing E. coli challenged weaned piglets
Abstract
Background: Post-weaned piglets suffer from F18+ Escherichia coli (E. coli) infections resulting in post-weaning diarrhoea or oedema disease. Frequently used management strategies, including colistin and zinc oxide, have contributed to the emergence and spread of antimicrobial resistance. Novel antimicrobials capable of directly interacting with pathogens and modulating the host immune responses are being investigated. Lactoferrin has shown promising results against porcine enterotoxigenic E. coli strains, both in vitro and in vivo.
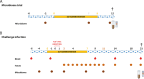
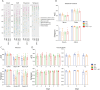
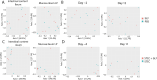
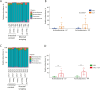
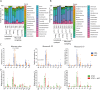
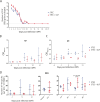
Results: We investigated the influence of bovine lactoferrin (bLF) on the microbiome of healthy and infected weaned piglets. Additionally, we assessed whether bLF influenced the immune responses upon Shiga toxin-producing E. coli (STEC) infection. Therefore, 2 in vivo trials were conducted: a microbiome trial and a challenge infection trial, using an F18+ STEC strain. BLF did not affect the α- and β-diversity. However, bLF groups showed a higher relative abundance (RA) for the Actinobacteria phylum and the Bifidobacterium genus in the ileal mucosa. When analysing the immune response upon infection, the STEC group exhibited a significant increase in F18-specific IgG serum levels, whereas this response was absent in the bLF group.
Conclusion: Taken together, the oral administration of bLF did not have a notable impact on the α- and β-diversity of the gut microbiome in weaned piglets. Nevertheless, it did increase the RA of the Actinobacteria phylum and Bifidobacterium genus, which have previously been shown to play an important role in maintaining gut homeostasis. Furthermore, bLF administration during STEC infection resulted in the absence of F18-specific serum IgG responses.
Keywords: E. coli; Immune modulation; Lactoferrin; Microbiome.
© 2024. The Author(s).
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. The manuscript has been read and approved by all named authors.
Figures
Similar articles
-
Porcine and Bovine Forms of Lactoferrin Inhibit Growth of Porcine Enterotoxigenic Escherichia coli and Degrade Its Virulence Factors.Appl Environ Microbiol. 2020 Nov 24;86(24):e00524-20. doi: 10.1128/AEM.00524-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32631861 Free PMC article.
-
Immunization of pregnant cows with Shiga toxin-2 induces high levels of specific colostral antibodies and lactoferrin able to neutralize E. coli O157:H7 pathogenicity.Vaccine. 2018 Mar 20;36(13):1728-1735. doi: 10.1016/j.vaccine.2018.02.060. Epub 2018 Feb 23. Vaccine. 2018. PMID: 29483033
-
Effects of a blend of essential oils, medium-chain fatty acids, and a toxin-adsorbing mineral on diarrhea and gut microbiome of weanling pigs experimentally infected with a pathogenic Escherichia coli.J Anim Sci. 2022 Jan 1;100(1):skab365. doi: 10.1093/jas/skab365. J Anim Sci. 2022. PMID: 34919701 Free PMC article.
-
Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: a global overview.J Infect. 2019 Aug;79(2):75-94. doi: 10.1016/j.jinf.2019.05.018. Epub 2019 May 28. J Infect. 2019. PMID: 31150744 Review.
-
Role of Recent Therapeutic Applications and the Infection Strategies of Shiga Toxin-Producing Escherichia coli.Front Cell Infect Microbiol. 2021 Jun 29;11:614963. doi: 10.3389/fcimb.2021.614963. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268129 Free PMC article. Review.
Cited by
-
Lactoferrin impairs pathogen virulence through its proteolytic activity.Front Vet Sci. 2024 Aug 8;11:1428156. doi: 10.3389/fvets.2024.1428156. eCollection 2024. Front Vet Sci. 2024. PMID: 39176399 Free PMC article. Review.
-
Shiga Toxin: Emerging Producer Strains, Prophylactic Approaches, and Application in Cancer Therapy.J Cancer Prev. 2024 Dec 30;29(4):120-128. doi: 10.15430/JCP.24.010. J Cancer Prev. 2024. PMID: 39790227 Free PMC article. Review.
References
-
- Fairbrother JM, Nadeau É. Diseases of Swine. 2019. Chapter 52: Colibacillosis; pp. 807–34.
-
- Barth S, Tscholshiew A, Menge C, Weiss R, Baljer G, Bauerfeind R. Virulence and fitness gene patterns of Shiga toxin-encoding Escherichia coli isolated from pigs with edema disease or diarrhea in Germany. Berl Munch Tierarztl Wochenschr. 2007;120(7–8):307–16. - PubMed
-
- Luppi A, Gibellini M, Gin T, Vangroenweghe F, Vandenbroucke V, Bauerfeind R, Bonilauri P, Labarque G, Hidalgo A. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porcine Health Manag. 2016;2:20. doi: 10.1186/s40813-016-0039-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

