Tibial cortex transverse transport promotes ischemic diabetic foot ulcer healing via enhanced angiogenesis and inflammation modulation in a novel rat model
- PMID: 38449025
- PMCID: PMC10918950
- DOI: 10.1186/s40001-024-01752-4
Tibial cortex transverse transport promotes ischemic diabetic foot ulcer healing via enhanced angiogenesis and inflammation modulation in a novel rat model
Abstract
Background: Tibial Cortex Transverse Transport (TTT) represents an innovative surgical method for treating lower extremity diabetic foot ulcers (DFUs), yet its underlying mechanisms remain elusive. Establishing an animal model that closely mirrors clinical scenarios is both critical and novel for elucidating the mechanisms of TTT.
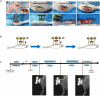
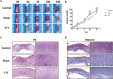
Methods: We established a diabetic rat model with induced hindlimb ischemia to mimic the clinical manifestation of DFUs. TTT was applied using an external fixator for regulated bone movement. Treatment efficacy was evaluated through wound healing assessments, histological analyses, and immunohistochemical techniques to elucidate biological processes.
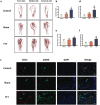
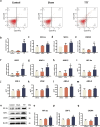
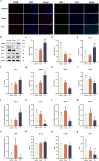
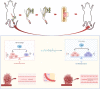
Results: The TTT group demonstrated expedited wound healing, improved skin tissue regeneration, and diminished inflammation relative to controls. Marked neovascularization and upregulation of angiogenic factors were observed, with the HIF-1α/SDF-1/CXCR4 pathway and an increase in EPCs being pivotal in these processes. A transition toward anti-inflammatory M2 macrophages indicated TTT's immunomodulatory capacity.
Conclusion: Our innovative rat model effectively demonstrates the therapeutic potential of TTT in treating DFUs. We identified TTT's roles in promoting angiogenesis and modulating the immune system. This paves the way for further in-depth research and potential clinical applications to improve DFU management strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

