Adverse Effects After Prehospital Administration of Naloxone by Bystanders: A Preliminary Study
- PMID: 38449098
- PMCID: PMC11035918
- DOI: 10.1017/S1049023X24000128
Adverse Effects After Prehospital Administration of Naloxone by Bystanders: A Preliminary Study
Abstract
Objective: Opioid use disorder is a cause of significant morbidity and mortality. In order to reverse opioid overdose as quickly as possible, many institutions and municipalities have encouraged people with no professional medical training to carry and administer naloxone. This study sought to provide preliminary data for research into the rates of adverse effects of naloxone when administered by bystanders compared to Emergency Medical Services (EMS) personnel, since this question has not been studied previously.
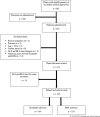
Methods: This was a retrospective cohort study performed at an urban, tertiary, academic medical center that operates its own EMS service. A consecutive sample of patients presenting to EMS with opioid overdose requiring naloxone was separated into two groups based on whether naloxone was administered by bystanders or by EMS personnel. Each group was analyzed to determine the incidence of four pre-specified adverse events.
Results: There was no significant difference in the rate of adverse events between the bystander (19%) and EMS (16%) groups (OR = 1.23; 95% CI, 0.63 - 2.32; P = .499) in this small sample. Based on these initial results, a study would need a sample size of 6,188 in order to reach this conclusion with 80% power. Similarly, there were no significant differences in the rates of any of the individual adverse events. Secondary analysis of patients' demographics showed differences between the two groups which generate hypotheses for further investigation of disparities in naloxone administration.
Conclusions: This preliminary study provides foundational data for further investigation of naloxone administration by bystanders. Adverse events after the prehospital administration of naloxone are rare, and future studies will require large sample sizes. These preliminary data did not demonstrate a statistically significant difference in adverse event rates when comparing naloxone administration by bystanders and EMS clinicians. This study provides data that will be useful for conducting further research on multiple facets of this topic.
Keywords: bystander; naloxone; opiate; opioid; overdose.
Conflict of interest statement
The authors declare none.
Figures
References
-
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS): CDC’s National Center for Injury Prevention and Control. www.cdc.gov/injury/wisqars. Accessed June 15, 2023.
-
- Ahmad FB, Cisewski JA, Rossen LM, et al. Provisional drug overdose death counts. National Center for Health Statistics. 2023. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed June 15, 2023.
-
- Ashburn NP, Ryder CW, Angi RM, et al. One-year mortality and associated factors in patients receiving out-of-hospital naloxone for presumed opioid overdose. Ann Emerg Med. 2020;75(5):559–567. - PubMed
-
- FDA Approves First Over-the-Counter Naloxone Nasal Spray. FDA.gov. Published online March 29, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-o.... Accessed June 15, 2023.
-
- Briefing Document, Joint Meeting of the Nonprescription Drug and the Anesthetic and Analgesic Drug Products Advisory Committees. Topic: Rx-to-OTC switch for NARCAN (naloxone HCl) Nasal Spray 4 mg, NDA 208411, Meeting Date: February 15, 2023. https://www.fda.gov/media/165339/download. Accessed June 15, 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical