Investigation of Neuroprotective Efficacy of Dexpanthenol in an Experimental Head Injury Model
- PMID: 38449284
- PMCID: PMC11375073
- DOI: 10.3340/jkns.2023.0219
Investigation of Neuroprotective Efficacy of Dexpanthenol in an Experimental Head Injury Model
Abstract
Objective: Dexpanthenol (DXP), which has known neuroprotective effects, has been shown to be beneficial in various experimental models and ischaemic diseases. The aim of this study was to investigate the possible neuroprotective effects of DXP in a traumatic brain injury (TBI) model.
Methods: Thirty-six Wistar-Albino female rats, approximately 6 months old, weighing 220-285 g were used. All rats were subjected to closed head trauma by dropping a weight of 350 g on the parietal region from a height of 50 cm at an angle of 180 degrees in the prepared head trauma model setup. The rats were divided into four groups as control (group 1), trauma (group 2), trauma + DXP (group 3), and DXP (group 4). In group 3, DXP was administered intraperitoneally at a dose of 500 mg/kg for six times at 30 minutes, 6, 12, 24, 36, and 48 hours. In group 4, DXP was administered intraperitoneally simultaneously with group 3 without causing head trauma. Blood samples were taken from all rats 72 hours later for biochemical examination. After blood samples were taken, rats were decapitated under general anaesthesia. Cerebral tissue samples were taken from decapitated rats for immunohistochemical and histopathological examination.
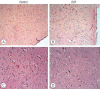
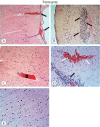
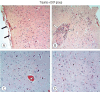
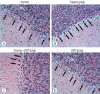
Results: Cytokine markers were found to be increased in posttraumatic brain tissue. Malondialdehyde and glutathione reductase levels were lower in group 3 compared to group 2. In addition, superoxide dismutase, glutathione peroxidase and catalase levels were significantly higher in group 3 compared to group 2. In histological evaluation, congestion in the piamater layer, cell infiltration, vascular congestion, hemorrhage and neuronal degeneration were significantly decreased in group 3 compared to group 2. DXP seems to be beneficial in neurological recovery in terms of histological and oxidative changes after head trauma in rats.
Conclusion: DXP should be further evaluated for its possible therapeutic effect in TBI.
Keywords: Dexpanthenol; Neuroprotection; Traumatic brain injury.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. - PubMed
-
- Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;5:1027–1039. discussion 1039-1040. - PubMed
-
- Binder S, Corrigan JD, Langlois JA. The public health approach to traumatic brain injury: an overview of CDC’s research and programs. J Head Trauma Rehabil. 2005;20:189–195. - PubMed
-
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. - PubMed
LinkOut - more resources
Full Text Sources

