A Pilot Study of Pembrolizumab Combined With Stereotactic Ablative Radiotherapy for Patients With Advanced or Metastatic Sarcoma
- PMID: 38449377
- PMCID: PMC10919132
- DOI: 10.1177/10732748241237331
A Pilot Study of Pembrolizumab Combined With Stereotactic Ablative Radiotherapy for Patients With Advanced or Metastatic Sarcoma
Abstract
Objectives: Immunotherapy with immune checkpoint inhibitors has shown only limited success in the management of metastatic soft tissue sarcoma. Overall response rates (ORR) with single agent pembrolizumab were 18% and median PFS was 18 weeks on the clinical trial SARC028. One strategy to improve the responses to immunotherapy is with stereotactic body radiation therapy (SBRT), which can enhance the antitumor CD8 T cell response through the release of tumor-specific antigens, potentially priming a more diverse class of T cell receptors.
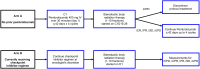
Methods: This is a phase 0, pilot prospective study taking place at a single center with 2 arms. In Arm A, patients are treated with pembrolizumab 400 mg IV infusion on day 1 of a 42-day cycle. Stereotactic body radiation therapy (SBRT) is delivered in 1-5 fractions starting on C1D15-28 and given every other day. In Arm B, patients who have started an immune checkpoint inhibitor within 60 days are treated with SBRT in addition to the current therapy.
Results: In this study we outline testing the feasibility of adding SBRT to pembrolizumab.
Conclusion: The ultimate goal of combination therapy is improved overall response, including tumors not treated with SBRT. This trial can be found registered online: NCT05488366.
Keywords: immunotherapy; pembrolizumab; radiation; sarcoma; stereotactic body radiation therapy.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397-1410. doi:10.1016/S1470-2045(17)30622-8 - DOI - PMC - PubMed
-
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415-423. doi:10.1016/S1470-2045(14)70063-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials